Is It Possible to Have Home E-Monitoring of Pulmonary Function in Our Patients with Duchenne Muscular Dystrophy in the COVID-19 Pandemic?—A One Center Pilot Study
Abstract
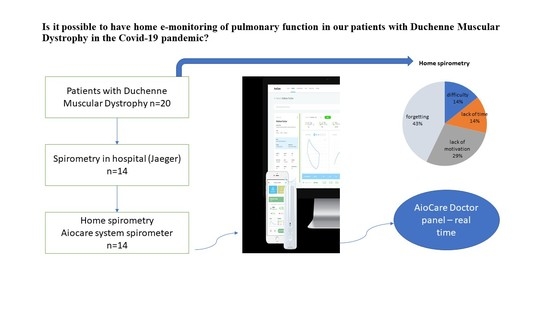
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Pulmonary Function Tests
2.3.1. Hospital Spirometry
2.3.2. Home Electronic Spirometry
2.4. Satisfaction Survey
2.5. Stage of Disease (Vignos Scale, Brooke Scale)
2.6. Statistical Analysis
3. Results
3.1. Participants
3.2. Pulmonary Function Test
3.2.1. Home Spirometry Frequency and Correctness
3.2.2. Hospital vs. Home Spirometry
3.2.3. Regression Analysis
3.3. Survey Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Birnkrant, D.J.; Bushby, K.; Bann, C.M.; Apkon, S.D.; Blackwell, A.; Brumbaugh, D.; Case, E.L.; Clemens, P.R.; Hadjiyannakis, S.; Pandya, S.; et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: Diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. Lancet Neurol. 2018, 17, 251–267. [Google Scholar] [CrossRef] [Green Version]
- Birnkrant, D.J.; Bushby, K.; Bann, C.; Alman, A.B.; Apkon, S.D.; Blackwell, A.; Case, L.; Cripe, L.; Hadjiyannakis, S.; Olson, A.K.; et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: Respiratory, cardiac, bone health, and orthopaedic management. Lancet Neurol. 2018, 17, 347–361. [Google Scholar] [CrossRef] [Green Version]
- LoMauro, A.; Romei, M.; Gandossini, S.; Pascuzzo, R.; Vantini, S.; D’Angelo, M.G.; Aliverti, A. Evolution of respiratory function in Duchenne muscular dystrophy from childhood to adulthood. Eur. Respir. J. 2018, 51, 1701418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wasilewska, E.; Małgorzewicz, S.; Meyer-Szary, J.; Sledzinska, K.; Niedoszytko, M.B.; Jassem, E.; Wierzba, J. Pulmonary dysfunction in children with Duchenne muscular dystrophy may appear earlier than we thought—Analysis using novel methodology based on z-scores. Arch. Med. Sci. 2021. [Google Scholar] [CrossRef]
- Finder, J.D.; Birnkrant, D.; Carl, J.; Farber, H.J.; Gozal, D.; Iannaccone, S.T.; Kovesi, T.; Kravitz, R.M.; Panitch, H.; Schramm, C.; et al. Respiratory Care of the Patient with Duchenne Muscular Dystrophy. Am. J. Respir. Crit. Care Med. 2004, 170, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.F.; Quinlivan, R.C.M.; Edwards, R.H.T.; Calverley, P.M.A. Changes in Spirometry Over Time as a Prognostic Marker in Patients with Duchenne Muscular Dystrophy. Am. J. Respir. Crit. Care Med. 2001, 164, 2191–2194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rideau, Y.; Jankowski, L.W.; Grellet, J. Respiratory function in the muscular dystrophies. Muscle Nerve 1981, 4, 155–164. [Google Scholar] [CrossRef]
- Gauld, L.M.; Boynton, A. Relationship between peak cough flow and spirometry in Duchenne muscular dystrophy. Pediatr. Pulmonol. 2005, 39, 457–460. [Google Scholar] [CrossRef]
- Beesley, V.; Molloy, H.; Elliott, M.W.; Ghosh, D. Non-invasive ventilation (NIV) in Duchenne muscular dystrophy: A review of 17 years of practice. Eur. Respir. J. 2016, 48, PA3067. [Google Scholar] [CrossRef]
- Pasnick, S.; Carlos, W.G.; Dela Cruz, C.S.; Gross, J.E.; Garrison, G.; Jamil, S. SARS-CoV-2 transmission and the risk of aerosol generating procedures. Am. J. Respir. Crit. Care. Med. 2020, 202, P13–P14. [Google Scholar] [CrossRef]
- European Respiratory Society. Recommendation from ERS Group 9.1 (Respiratory Function Technologists/Scientists) Lung Function Testing during COVID-19 Pandemic and Beyond. 2020. Available online: www.ersnet.org/covid-19-guidelines-and-recommendations-directory (accessed on 15 August 2020).
- American Thoracic Society. Pulmonary Function Laboratories: Advice Regarding COVID-19. 2020. Available online: www.thoracic.org/professionals/clinical-resources/disease-related-resources/pulmonary-function-laboratories.php (accessed on 20 June 2020).
- Poberezhets, V.; Pinnock, H.; Vogiatzis, I.; Mishlanov, V. Implementation of digital health interventions in respiratory medicine: A call to action by the European Respiratory Society m-Health/e-Health Group. ERJ Open Res. 2020, 6, 00281–02019. [Google Scholar] [CrossRef] [Green Version]
- ERS Rochester, C.L.; Vogiatzis, I.; Holland, A.E.; Lareau, S.C.; Marciniuk, D.D.; Puhan, M.A.; Spruit, M.A.; Masefield, S.; Casaburi, R.; Clini, E.M.; et al. An Official American Thoracic Society/European Respiratory Society Policy Statement: En-hancing Implementation, Use, and Delivery of Pulmonary Rehabilitation. Am. J. Respir. Crit. Care Med. 2015, 192, 1373–1386. [Google Scholar] [CrossRef] [Green Version]
- Veerapandiyan, A.; Wagner, K.R.; Apkon, S.; McDonald, C.M.; Mathews, K.D.; Parsons, J.A.; Wong, B.L.; Eichinger, K.; Shieh, P.B.; Butterfield, R.J.; et al. The care of patients with Duchenne, Becker, and other muscular dystrophies in the COVID-19 pandemic. Muscle Nerve 2020, 62, 41–45. [Google Scholar] [CrossRef]
- Aiocare. Available online: https://healthcloud.aiocare.com (accessed on 1 January 2020).
- Sobierajska-Rek, A.; Mański, Ł.; Jabłońska-Brudło, J.; Śledzińska, K.; Wasilewska, E.; Szalewska, D. Respiratory Telerehabilitation of Boys and Young Men with Duchenne Muscular Dystrophy in the COVID-19 Pandemic. Int. J. Environ. Res. Public Health 2021, 18, 6179. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.R.; Crapo, R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Enright, P.; van der Grinten, C.P.M.; Gustafsson, P.; et al. General considerations for lung function testing. Eur. Respir. J. 2005, 26, 153–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vignos, J.P.J.; Archibald, K.C. Maintenance of ambulation in childhood muscular dystrophy. J. Chronic Dis. 1960, 12, 273–290. [Google Scholar] [CrossRef]
- Brooke, M.H.; Griggs, R.C.; Mendell, J.R.; Fenichel, G.M.; Shumate, J.B.; Pellegrino, R.J. Clinical trial in Duchenne dystrophy. The design of the protocol. Muscle Nerve 1981, 4, 186–197. [Google Scholar] [CrossRef]
- Franczuk, M.; Przybyłowski, T.; Czajkowska-Malinowska, M.; Radliński, J.; Bochenek, G.; Wesołowski, S.; Sliwiński, P. Spirometry during the SARS-CoV-2 pandemic. Guidelines and practical advice from the expert panel of Respiratory Physiopathology Assembly of Polish Respiratory Society. Adv. Respir. Med. 2020, 88, 640–650. [Google Scholar] [CrossRef]
- Kupczyk, M.; Hofman, A.; Kołtowski, Ł.; Kuna, P.; Łukaszyk, M.; Buczyłko, K.; Bodzenta-Łukaszyk, A.; Nastałek, P.; Soliński, M.; Dąbrowiecki, P. Home self-monitoring in patients with asthma using a mobile spirometry system. J. Asthma 2020, 58, 505–511. [Google Scholar] [CrossRef] [Green Version]
- Hofman, A.; Kupczyk, M.; Kuna, P. Application of the AioCare system in monitoring exacerbations of bronchial asthma. Pol. J. Allergol. Spec. Issues 2018, 1, A.8. (In Polish) [Google Scholar]
- Jankowski, P.; Górska, K.; Mycroft, K.; Korczyński, P.; Soliński, M.; Kołtowski, Ł.; Krenke, R. The use of a mobile spirometry with a feedback quality assessment in primary care setting—A nationwide cross-sectional feasibility study. Respir. Med. 2021, 184, 106472. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowiecki, P.; Adamkiewicz, Ł.; Mucha, D.; Czechowski, P.O.; Soliński, M.; Chciałowski, A.; Badyda, A. Impact of Air Pollution on Lung Function among Preadolescent Children in Two Cities in Poland. J. Clin. Med. 2021, 10, 2375. [Google Scholar] [CrossRef]
- Wasilewska, E.; Małgorzewicz, S.; Sobierajska-Rek, A.; Jabłońska-Brudło, J.; Górska, L.; Śledzińska, K.; Bautembach-Minkowska, J.; Wierzba, J. Transition from Childhood to Adulthood in Patients with Duchenne Muscular Dystrophy. Medicina 2020, 56, 426. [Google Scholar] [CrossRef] [PubMed]
- Buyse, G.M.; Rummey, C.; Meier, T.; Leinonen, M.; Voit, T.; McDonald, C.M.; Mayer, O.H. Home-Based Monitoring of Pulmonary Function in Patients with Duchenne Muscular Dystroph. J. Neuromuscul. Dis. 2018, 5, 419–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO Global Observatory for eHealth. Telemedicine: Opportunities and Developments in Member States: Report on the Second Global Survey on eHealth. World Health Organization, 2010. Available online: https://apps.who.int/iris/handle/10665/44497 (accessed on 15 August 2020).



| ID | Age | Weight | Height | BMI | AS | VS | BS | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Years | kg | Centile | cm | Centile | Centile | |||||
| 1 | 13 | 34 | 3 | 137 | 1 | 18.1 | 40 | 1 | 2 | 6 |
| 2 | 10 | 24.5 | 1 | 119 | 1 | 17.3 | 48 | 1 | 2 | 3 |
| 3 | 14 | 57 | 52 | 145 | 1 | 27.1 | 96 | 1 | 3 | 4 |
| 4 | 15 | 90 | 97 | 181 | 80 | 27.5 | 97 | 0 | 9 | 4 |
| 5 | 15 | 82 | 97 | 160 | 1 | 32.0 | 99 | 0 | 8 | 5 |
| 6 | 11 | 55.5 | 88 | 150.5 | 40 | 24.5 | 94 | 1 | 1 | 6 |
| 7 | 10 | 42 | 76 | 142 | 38 | 20.8 | 86 | 0 | 8 | 6 |
| 8 | 10 | 50 | 93 | 140 | 34 | 25.5 | 97 | 0 | 8 | 6 |
| 9 | 11 | 48 | 76 | 133 | 16 | 27.1 | 91 | 1 | 2 | 5 |
| 10 | 16 | 64 | 79 | 170 | 64 | 22.1 | 81 | 0 | 9 | 1 |
| 11 | 10 | 56 | 95 | 141 | 28 | 28.2 | 98 | 0 | 9 | 5 |
| 12 | 9 | 43 | 88 | 138 | 41 | 22.6 | 93 | 1 | 1 | 2 |
| 13 | 16 | 49 | 8 | 160 | 2 | 19.1 | 34 | 1 | 1 | 1 |
| 14 | 15 | 70 | 70 | 166 | 7 | 25.4 | 91 | 0 | 9 | 2 |
| Mean ± SD/Median (IQR) | 12.5 ± 2.6 | 54.7 ± 17.6 | 65.9 ± 35.7 | 148.9 ± 16.7 | 25.3 ± 25.6 | 24.1 ± 4.3 | 81.8 ± 23.0 | - | 4.9 ± 3.4 | 4.0 ± 1.9 |
| Home Spirometry | |||||
|---|---|---|---|---|---|
| ID | Days of Measurements | Total Measurements | Acceptable Measurements | ||
| nb | % | nb | nb | % | |
| 1 | 10.0 | 35.7 | 11 | 8 | 73 |
| 2 | 28.0 | 100.0 | 57 | 0 | 0 |
| 3 | 3.0 | 10.7 | 3 | 0 | 0 |
| 4 | 3.0 | 10.7 | 3 | 0 | 0 |
| 5 | 8.0 | 28.6 | 11 | 0 | 0 |
| 6 | 28.0 | 100.0 | 45 | 0 | 0 |
| 7 | 21.0 | 75.0 | 27 | 0 | 0 |
| 8 | 26.0 | 92.8 | 28 | 21 | 75 |
| 9 | 7.0 | 25.0 | 12 | 3 | 25 |
| 10 | 8.0 | 28.6 | 12 | 2 | 17 |
| 11 | 7.0 | 25.0 | 10 | 0 | 0 |
| 12 | 7.0 | 25.0 | 8 | 0 | 0 |
| 13 | 19.0 | 67.9 | 27 | 5 | 19 |
| 14 | 23.0 | 82.1 | 29 | 5 | 17 |
| Mean ± SD | 14.1 ± 9.5 | 50.5 ± 33.8 | 20.2 ± 16.0 | 3.1 ± 5.7 | 16.1 ± 26.1 |
| ID | Home (AioCare) Spirometry | Hospital (Jaeger) Spirometry | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FVC (L) | FEV1 (L) | PEF (L/min) | FVC (%) | FEV1 (%) | PEF (%) | FVC (L) | FEV1 (L) | PEF (L/min) | FVC (%) | FEV1 (%) | PEF (%) | |
| 1 | 2.07 ± 0.05 | 1.80 ± 0.07 | 243 ± 10 | 79.9 ± 1.7 | 77.7 ± 2.8 | 83.5 ± 3.6 | 1.73 ± 0.22 | 1.44 ± 0.71 | 208 ± 1.33 | 77.4 ± 0.43 | 76.9 ± 0.41 | 80.7 ± 0.45 |
| 2 | 1.05 ± 0.30 | 0.90 ± 0.19 | 86 ± 27 | 62.3 ± 17.8 | 60.2 ± 12.6 | 41.2 ± 12.9 | 1.22 ± 0.42 | 1.2 ± 0.45 | 98 ± 6.55 | 87.1 ± 2.25 | 98.7 ± 1.44 | 52.8 ± 4.31 |
| 3 | 1.79 ± 0.33 | 1.55 ± 0.24 | 165 ± 2 | 60.4 ± 11.0 | 59.1 ± 9.0 | 50.3 ± 0.5 | 2.22 ± 0.32 | 1.79 ± 0.43 | 244 ± 2.2 | 84.1 ± 0.41 | 81.2 ± 0.23 | 83.0 ± 0.33 |
| 4 | 2.25 ± 1.84 | 1.83 ± 1.58 | 170 ± 144 | 43.2 ± 35.3 | 41.5 ± 35.8 | 32.5 ± 27.7 | 2.94 ± 0.34 | 2.63 ± 0.9 | 333 ± 17 | 65.5 ± 0.34 | 69.7 ± 2.3 | 69.6 ± 3.5 |
| 5 | 4.02 ± 1.97 | 2.22 ± 0.52 | 186 ± 43 | 106.9 ± 52.3 | 67.8 ± 15.9 | 38.3 ± 8.9 | 2.82 ± 1.22 | 2.09 ± 0.21 | 212 ± 14 | 87.5 ± 0.93 | 76.5 ± 1.32 | 57.3 ± 2.11 |
| 6 | 2.78 ± 0.19 | 2.59 ± 0.15 | 332 ± 14 | 97.0 ± 6.5 | 106.7 ± 6.4 | 93.4 ± 3.8 | 2.76 ± 0.55 | 2.49 ± 0.65 | 321 ± 11 | 101.8 ± 3.21 | 107.7 ± 2.54 | 100.1 ± 1.54 |
| 7 | 2.32 ± 0.23 | 2.05 ± 0.20 | 195 ± 35 | 90.4 ± 9.0 | 93.9 ± 9.0 | 62.1 ± 11.2 | 2.13 ± 0.34 | 1.92 ± 0.31 | 231 ± 11 | 85.7 ± 0.34 | 92.1 ± 2.11 | 82.6 ± 2.57 |
| 8 | 2.49 ± 0.61 | 2.14 ± 0.18 | 233 ± 19 | 111.8 ± 27.4 | 110.8 ± 9.1 | 76.5 ± 6.2 | 2.13 ± −0.43 | 1.86 ± 0.54 | 232 ± 14 | 89.4 ± 2.12 | 93.1 ± 3.20 | 85.5 ± 3.5 |
| 9 | 1.40 ± 0.42 | 1.00 ± 0.31 | 145 ± 49 | 70.8 ± 21.1 | 58.4 ± 17.9 | 53.1 ± 17.8 | 1.58 ± 0.55 | 1.15 ± 0.45 | 215 ± 12 | 77.1 ± 1.32 | 66.9 ± 2.54 | 89.6 ± 6.23 |
| 10 | 2.14 ± 0.48 | 1.81 ± 0.22 | 228 ± 53 | 50.7 ± 11.3 | 49.7 ± 6.0 | 44.1 ± 10.3 | 2.00 ± 0.41 | 1.98 ± 0.92 | 233 ± 16 | 47.5 ± 2.40 | 56.8 ± 3.13 | 54.6 ± 3.22 |
| 11 | 1.62 ± 0.42 | 1.06 ± 0.25 | 110 ± 26 | 72.5 ± 19.1 | 55.0 ± 12.7 | 35.5 ± 8.4 | 1.38 ± 0.74 | 1.13 ± 0.34 | 115 ± 14 | 56.6 ± 2.43 | 55.5 ± 1.34 | 41.6 ± 3.44 |
| 12 | 2.04 ± 0.10 | 1.84 ± 0.09 | 206 ± 32 | 91.4 ± 4.7 | 95.2 ± 4.5 | 68.6 ± 10.6 | 1.94 ± 0.75 | 1.70 ± 0.39 | 187 ± 31 | 85.0 ± 2.4 | 88.8 ± 4.1 | 71.6 ± 3.5 |
| 13 | 2.63 ± 0.24 | 2.39 ± 0.27 | 243 ± 41 | 69.9 ± 6.3 | 73.2 ± 8.3 | 50.2 ± 8.5 | 2.74 ± 0.34 | 2.48 ± 0.49 | 258 ± 23 | 77.6 ± 2,4 | 84.5 ± 5.2 | 69.8 ± 5.43 |
| 14 | 2.96 ± 0.14 | 2.52 ± 0.12 | 249 ± 13 | 70.0 ± 3.4 | 69.0 ± 3.3 | 48.8 ± 2.6 | 2.76 ± 0.45 | 2.39 ± 0.34 | 238 ± 32 | 70.0 ± 3.2 | 73.3 ± 1.55 | 59.0 ± 3.56 |
| Home (AioCare) Spirometry %pv | Hospital (Jaeger) Spirometry %pv | Mean Difference Home vs. Hospital Spirometry | p-Value * | Correlation r ** | |
|---|---|---|---|---|---|
| FVC (L) | 2.25 ± 0.73 | 2.17 ± 0.57 | 0.09 ± 0.44 | 0.476 | 0.80 |
| FEV1 (L) | 1.84 ± 0.55 | 1.88 ± 0.51 | −0.04 ± 0.29 | 0.624 | 0.85 |
| PEF (L/min) | 199 ± 63 | 223 ± 64 | −23.86 ± 51.32 | 0.106 | 0.67 |
| FVC%pv | 76.94 ± 20.36 | 78.02 ± 14.21 | −1.08 ± 15.17 | 0.795 | 0.67 |
| FEV1%pv | 72.72 ± 21.47 | 80.12 ± 15.23 | −7.40 ± 14.43 | 0.077 | 0.74 |
| PEF%pv | 55.57 ± 18.68 | 71.27 ± 16.58 | −15.70 ± 12.47 | 0.0004 | 0.76 |
| Model 1 Age, BMI, AS, VS, BS, FEV1%pv | ||
| AIC: | 101.00 | |
| Variables: | Coefficient (95%CI) | p-value |
| Intercept | 14.69 (−15.64–45.03) | 0.3628 |
| BMI | −0.73 (−1.71–0.26) | 0.1744 |
| FEV1%pv | 0.234 (0.039–0.430) | 0.0387 |
| Model 2: age, BMI, AS, VS, BS, FVC%pv | ||
| AIC: | 102.19 | |
| Variables | Coefficient (95%CI) | p-value |
| Intercept | 24.71 (−2.81–52.22) | 0.1062 |
| BMI | −1.45 (−2.17–−0.13) | 0.0494 |
| FVC%pv | 0.2226 (0.0090–0.4362) | 0.0659 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wasilewska, E.; Sobierajska-Rek, A.; Małgorzewicz, S.; Soliński, M.; Szalewska, D.; Jassem, E. Is It Possible to Have Home E-Monitoring of Pulmonary Function in Our Patients with Duchenne Muscular Dystrophy in the COVID-19 Pandemic?—A One Center Pilot Study. Int. J. Environ. Res. Public Health 2021, 18, 8967. https://doi.org/10.3390/ijerph18178967
Wasilewska E, Sobierajska-Rek A, Małgorzewicz S, Soliński M, Szalewska D, Jassem E. Is It Possible to Have Home E-Monitoring of Pulmonary Function in Our Patients with Duchenne Muscular Dystrophy in the COVID-19 Pandemic?—A One Center Pilot Study. International Journal of Environmental Research and Public Health. 2021; 18(17):8967. https://doi.org/10.3390/ijerph18178967
Chicago/Turabian StyleWasilewska, Eliza, Agnieszka Sobierajska-Rek, Sylwia Małgorzewicz, Mateusz Soliński, Dominika Szalewska, and Ewa Jassem. 2021. "Is It Possible to Have Home E-Monitoring of Pulmonary Function in Our Patients with Duchenne Muscular Dystrophy in the COVID-19 Pandemic?—A One Center Pilot Study" International Journal of Environmental Research and Public Health 18, no. 17: 8967. https://doi.org/10.3390/ijerph18178967
APA StyleWasilewska, E., Sobierajska-Rek, A., Małgorzewicz, S., Soliński, M., Szalewska, D., & Jassem, E. (2021). Is It Possible to Have Home E-Monitoring of Pulmonary Function in Our Patients with Duchenne Muscular Dystrophy in the COVID-19 Pandemic?—A One Center Pilot Study. International Journal of Environmental Research and Public Health, 18(17), 8967. https://doi.org/10.3390/ijerph18178967