How Do Serum Zonulin Levels Change in Gestational Diabetes Mellitus, Pregnancy Cholestasis, and the Coexistence of Both Diseases?
Abstract
:1. Introduction
2. Materials and Methods
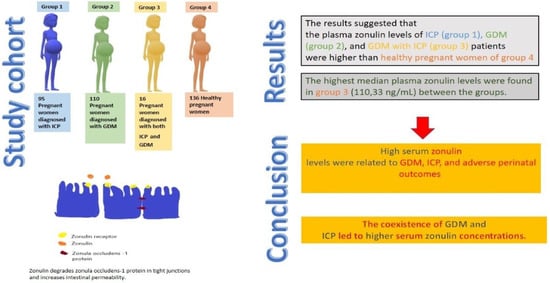
- Group 1: 95 pregnant women diagnosed with ICP;
- Group 2: 110 pregnant women diagnosed with GDM;
- Group 3: 16 women diagnosed with both GDM and ICP;
- Group 4: 136 healthy pregnant women as the control group.
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williamson, C.; Geenes, V. Intrahepatic Cholestasis of Pregnancy. Obstet. Gynecol. 2014, 124, 120–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, D.D.; Rood, K.M. Intrahepatic cholestasis of pregnancy. Clin. Obstet. Gynecol. 2020, 63, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Ovadia, C.; Seed, P.T.; Sklavounos, A.; Geenes, V.; Di Ilio, C.; Chambers, J.; Kohari, K.; Bacq, Y.; Bozkurt, N.; Brun-furrer, R.; et al. Association of adverse perinatal outcomes of intrahepatic cholestasis of pregnancy with biochemical markers: Results of aggregate and individual patient data meta-analyses. Lancet 2019, 393, 899–909. [Google Scholar] [CrossRef] [Green Version]
- Kim, C. Maternal outcomes and follow-up after gestational diabetes mellitus. Diabet. Med. 2015, 31, 292–301. [Google Scholar] [CrossRef] [Green Version]
- Gadaleta, R.M.; Erpecum, K.J.; Van Oldenburg, B.; Willemsen, E.C.L.; Renooij, W.; Murzilli, S.; Klomp, L.W.; Siersema, P.D.; Schipper, M.E.; Danese, S.; et al. Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. Gut 2011, 64, 463–472. [Google Scholar] [CrossRef]
- Gonzalez, F.; Jiang, C.; Xie, C.; Patterson, A. Intestinal Farnesoid X Receptor Signaling Modulates Metabolic Disease. Dig. Dis. 2019, 35, 178–184. [Google Scholar] [CrossRef]
- Martineau, M.; Raker, C.; Powrie, R.; Williamson, C. Intrahepatic cholestasis of pregnancy is associated with an increased risk of gestational diabetes. Eur. J. Obstet. Gynecol. 2014, 176, 80–85. [Google Scholar] [CrossRef]
- Arafa, A.; Dong, J.; Arafa, A. Association between intrahepatic cholestasis of pregnancy and risk of gestational diabetes and preeclampsia: A systematic review and meta-analysis. Hypertens. Pregnancy 2020, 39, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Fukui, H. Stomach and Colon: Review Increased Intestinal Permeability and Decreased Barrier Function: Does It Really Influence the Risk of Inflammation? Inflamm. Intest. Dis. 2016, 1, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, L.; Zheng, Y.; Yue, F.; Russell, R.D.; Zeng, Y. Circulating zonulin levels in newly diagnosed Chinese type 2 diabetes patients. Diabetes Res. Clin. Pract. 2014, 106, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Pijls, K.; Jonkers, D.; Elamin, E.; Masclee, A.; Koek, G. Intestinal epithelial barrier function in liver cirrhosis: An extensive review of the literature. Liver Int. 2013, 33, 1457–1469. [Google Scholar] [CrossRef]
- Mouries, J.; Brescia, P.; Adorini, L.; Rescigno, M. Microbiota-driven gut vascular barrier disruption is a prerequisite for non-alcoholic steatohepatitis development. J. Hepatol. 2019, 71, 1216–1228. [Google Scholar] [CrossRef] [Green Version]
- Fasano, A. Zonulin, regulation of tight junctions, and autoimmune diseases. Ann. N. Y. Acad. Sci. 2013, 1258, 25–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drago, S.; Asmar, R.E.L.; Pierro, M.D.I.; Clemente, M.G.; Tripathi, A.; Sapone, A.; Thakar, M.; Iacono, G.; Carroccio, A.; D’Agate, C.; et al. Gliadin, zonulin and gut permeability: Effects on celiac and non-celiac intestinal mucosa and intestinal cell lines. Scand. J. Gastroenterol. 2006, 41, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Reyes, H.; Zapata, R.; Hern, I.; Sandoval, L.; Silva, J.J. Is a Leaky Gut Involved in the Pathogenesis of Intrahepatic Cholestasis of Pregnancy? Hepatology 2006, 43, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Bawah, A.T.; Seini, M.M.; Yakubu, Y.A.; Ussher, F.A.; Amoah, B.Y.; Alidu, H. First trimester zonulin levels and adiposity as predictive indices of gestational diabetes mellitus. Int. J. Diabetes Dev. Ctries 2019, 39, 451–457. [Google Scholar] [CrossRef]
- Bicocca, M.J.; Sperling, J.D.; Chauhan, S.P. Intrahepatic cholestasis of pregnancy : Review of six national and regional guidelines. Eur. J. Obstet. Gynecol. 2018, 231, 180–187. [Google Scholar] [CrossRef]
- Roncaglia, N.; Locatelli, A.; Arreghini, A.; Assi, F.; Cameroni, I.; Pezzullo, J.C.; Ghindi, A. A randomised controlled trial of ursodeoxycholic acid and S-adenosyl-l-methionine in the treatment of gestational cholestasis. BJOG Int. J. Obstet. Gynaecol. 2004, 111, 17–21. [Google Scholar] [CrossRef] [Green Version]
- American Diabetes Association. Standards of Medical Care in Diabetes—2013. Diabetes Care 2013, 36 (Suppl. 1), 11–66. [Google Scholar] [CrossRef] [Green Version]
- Mokkala, K.; Tertti, K.; Rönnemaa, T.; Vahlberg, T.; Laitinen, K. Evaluation of serum zonulin for use as an early predictor for gestational diabetes. Nutr. Diabetes 2017, 7, 2016–2018. [Google Scholar] [CrossRef] [Green Version]
- Mokkala, K.; Pellonperä, O.; Röytiö, H.; Pussinen, P.; Rönnemaa, T.; Laitinen, K. Increased intestinal permeability, measured by serum zonulin, is associated with metabolic risk markers in overweight pregnant women. Metabolism 2017, 69, 43–50. [Google Scholar] [CrossRef] [Green Version]
- Demir, E.; Ozkan, H.; Seckin, K.D.; Sahtiyancı, B.; Demir, B. Plasma Zonulin Levels as a Non-Invasive Biomarker of Intestinal Permeability in Women with Gestational Diabetes Mellitus. Biomolecules 2019, 9, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deniz, C.D.; Ozler, S.; Sayın, F.K. Association of adverse outcomes of intrahepatic cholestasis of pregnancy with zonulin levels. J. Obstet. Gynaecol. (Lahore) 2020, 41, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Lammers, K.M.; Goldblum, S.; Shea-donohue, T.; Netzel-arnett, S.; Buzza, M.S.; Antalis, T.M.; Vogel, S.N.; Zhao, A.; Yang, S.; et al. Identification of human zonulin, a physiological modulator of tight junctions, as prehaptoglobin-2. Proc. Natl. Acad. Sci. USA 2009, 106, 16799–16804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fasona, A. All disease begins in the (leaky) gut: Role of zonulin-mediated gut permeability in the pathogenesis of some chronic inflammatory diseases. F1000 Res. 2020, 9. [Google Scholar] [CrossRef]
- Kosters, A.; Karpen, S.J. The role of inflammation in cholestasis–Clinical and basic aspects. Semin. Liver Dis. 2010, 30, 186–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zak-Golab, A.; Kocelak, P.; Aptekorz, M.; Zeintara, M.; Juszczyk, L.; Matirosian, G.; Chudek, J.; Olszanecka-Glinianowicz, M. Gut microbiota, microinflammation, metabolic profile, and zonulin concentration in obese and normal weight subjects. Int. J. Endocrinol. 2013, 2013, 674106. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.Y.; Shariff, Z.M.; Yosuf, B.N.M.; Rejali, Z.; Tee, Y.Y.S.; Bindels, J.; van Der Beek, E.M. Independent and combined effects of age, body mass index and gestational weight gain on the risk of gestational diabetes mellitus. Sci. Rep. 2020, 10, 8486. [Google Scholar] [CrossRef]
- Yule, C.S.; Holcomb, D.S.; Kraus, A.C.; Brown, C.E.L.; McIntre, D.D.; Nelson, D.B. Cholestasis: A prospective study of perinatal outcomes and time to symptom improvement. Am. J. Perinatol. 2021, 38, 414–420. [Google Scholar] [CrossRef]
- Aftab, N.; Faraz, S.; Hazari, K.; Mahgoub, F.B. Maternal and fetal outcome in intrahepatic cholestasis of pregnancy in a multicultural society conducted at a tertiary care hospital in Dubai. Dubai Med. J. 2021, 4, 53–59. [Google Scholar] [CrossRef]
- Singh, S.; Parihar, S. Perinatal outcomes and intrahepatic cholestasis of pregnancy: A prospective study. Int. J. Reprod. Contracept. Obstet. Gynecol. 2019, 8, 1177. [Google Scholar]
- Krstevska, S.S.; Nakova, V.V.; Samordziski, I.; Bosku, A.A.; Todarovska, I.; Sıma, A.; Livrinova, V.; Jovanovska, V.; Milkovski, D. Perinatal outcomes in gestational diabetes mellitus vs normoglisemic women. Biomed. J. Sci. Tech. Res. 2020, 26, 19882–19888. [Google Scholar]
- Makwana, M.; Bhimwal, R.K.; Ram, C.; Mathur, S.L.; Lal, K.; Mourya, H. Gestational diabetes mellitus with its maternal and foetal outcome: A clinical study. Int. J. Adv. Med. 2017, 4, 9919–9925. [Google Scholar] [CrossRef] [Green Version]
- Scheffler, L.; Crane, A.; Heyne, H.; Tönjes, A.; Schleinitz, D.; Ihling, C.; Stumvoll, M.; Freire, R.; Fiorentino, M.; Fasano, A.; et al. Widely used commercial ELISA does not detect precursor of haptoglobin2, but recognizes properdin as a potential second member of the zonulin family. Front. Endocrinonl. 2018, 9, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Variables | Group 1 (n = 88) | Group 2 (n = 91) | Group 3 (n = 16) | Group 4 (n = 136) | p Value |
|---|---|---|---|---|---|
| Age (years) median (min–max) | 26.5 (18–41) | 27 (20–42) | 32 (18–39) | 26 (18–45) | 0.127 a |
| BMI (kg/m2) median (min–max) | 27.71 (20.12–39.12) | 30.12 (22.12–38.12) | 35.39 (27.88–39.12) | 26.38 (20.22–42.12) | <0.001 a |
| Gravidity median (min–max) | 1 (1–6) | 1 (1–5) | 1 (1–6) | 2 (1–8) | 0.724 a |
| Parity median (min–max) | 0 (0–4) | 0 (0–4) | 0 (0–3) | 1 (0–5) | 0.710 a |
| Gestational week of delivery median (min–max) | 37 (34–40) | 39 (28–40) | 36 (34–38) | 39 (30–42) | <0.001 a |
| 1st minute APGAR score median (min–max) | 9 (5–9) | 8 (5–9) | 8 (5–9) | 9 (5–9) | <0.001 a |
| 5th minute APGAR score median (min–max) | 9 (8–10) | 10 (7–10) | 9 (8–10) | 10 (8–10) | <0.001 a |
| Zonulin (ng/mL) median (min–max) | 12.11 (1.77–149.12) | 31,03 (0.77–103.11) | 110.33 (66.12–188.9) | 4.77 (0.12–101.11) | <0.001 a |
| Delivery type (CS/VD) n (%) | 37/51 (42%/58%) | 31/60 (34.1%/65.9%) | 3/13 (18.8%/81.3%) | 27/109 (19.9%/80.1%) | 0.002 b |
| NICU admission n (%) | 7 (8%) | 19 (20.9%) | 3 (18.8%) | 8 (5.9%) | 0.003 b |
| Meconium staining n (%) | 8 (9.1%) | 10 (11%) | 8 (50%) | 9 (6.6%) | <0.001 b |
| Variables | Group 1–Group 2 | Group 1–Group 3 | Group 1–Group 4 | Group 2–Group 3 | Group 2–Group 4 | Group 3–Group 4 |
|---|---|---|---|---|---|---|
| BMI (kg/m2) | <0.001 | <0.001 | 0.520 | <0.001 | <0.001 | <0.001 |
| Gestational week of delivery | <0.001 | 0.001 | <0.001 | <0.001 | 0.933 | <0.001 |
| 1st minute APGAR score | <0.001 | 0.025 | <0.001 | 0.772 | <0.001 | <0.001 |
| 5th minute APGAR score | <0.001 | 0.209 | <0.001 | <0.001 | 0.973 | <0.001 |
| Zonulin | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Delivery type (CS) | 0.271 | 0.138 | <0.001 | 0.356 | 0.024 | 1 |
| NICU admission | 0.025 | 0.181 | 0.740 | 1 | 0.001 | 0.094 |
| Meconium staining | 0.862 | <0.001 | 0.671 | 0.001 | 0.357 | <0.001 |
| Groups | 2500 g–3999 g (n = 262) | ≥4000 g (n = 50) | <2500 g (n = 19) | p |
|---|---|---|---|---|
| Group 1 (n = 88) | 78 (88.6%) | 2 (2.3%) | 8 (9.1%) | <0.001 |
| Group 2 (n = 91) | 54 (59.3%) | 33 (36.3%) | 4 (4.4%) | |
| Group 3 (n = 16) | 10 (62.5%) | 1 (6.3%) | 5 (31.3%) | |
| Group 4 (n = 136) | 120 (88.2%) | 14 (10.3%) | 2 (1.5%) |
| Variables | Mild ICP (n = 80) | Severe ICP (n = 24) | p |
|---|---|---|---|
| Age (years) median (min–max) | 28 (18–41) | 29 (19–41) | 0.169 a |
| BMI (kg/m2) median (min–max) | 27.77 (20.12–39.12) | 29.11 (23.33–39.12) | 0.057 b |
| Gravidity median (min–max) | 1 (1–6) | 1,5 (1–6) | 0.166 b |
| Parity median (min–max) | 0 (0–3) | 0,5 (0–4) | 0.229 b |
| Gestational week of delivery median (min–max) | 38 (35–40) | 36 (34–37) | <0.001 b |
| 1. minute APGAR score median (min–max) | 9 (6–9) | 8 (5–9) | 0.032 b |
| 5. minute APGAR score median (min–max) | 9 (8–10) | 9 (8–10) | 0.404 b |
| Zonulin (ng/mL) | 11.84 (1.77–116.12) | 83.27 (5.81–188.9) | <0.001 b |
| Delivery type (CS/VD) n (%) | 33/47 (41.2%/58.8%) | 7/17 (29.2%/70.8%) | 0.408 c |
| NICU admission n (%) | 0 (0%) | 10 (41.7%) | <0.001 c |
| Meconium staining n (%) | 2 (2.5%) | 14 (58.3%) | <0.001 c |
| Birth weight (2500 g–3999 g /≥4000 g/S < 2500 g) n (%) | 75/3/2 (93.8%/3.8%/2.5%) | 13/0/11 (54.2%/0%/45.8%) | <0.001 c |
| BMI Levels | Group 1 | Group 2 | Group 3 | Group 4 | p Value |
|---|---|---|---|---|---|
| <25 | 12.24 (1.77–99.12) | 10.50 (6.00–15) | No data | 4.9 (10.12–23.88) | 0.601 |
| 25–29 | 11.13 (2.33–149.12) | 18.5 (2.33–101.20) | 108.12 (66.12–150,12) | 6.7 (0.16–150.12) | 0.015 |
| ≥30 | 15.09 (2.88–116.12) | 44.66 (0.77–103.11) | 110.33 (77.32–188.90) | 7.5 (0.26–101.11) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Güvey, H.; Çelik, S.; Çalışkan, C.S.; Yılmaz, Z.; Yılmaz, M.; Erten, Ö.; Tinelli, A. How Do Serum Zonulin Levels Change in Gestational Diabetes Mellitus, Pregnancy Cholestasis, and the Coexistence of Both Diseases? Int. J. Environ. Res. Public Health 2021, 18, 12555. https://doi.org/10.3390/ijerph182312555
Güvey H, Çelik S, Çalışkan CS, Yılmaz Z, Yılmaz M, Erten Ö, Tinelli A. How Do Serum Zonulin Levels Change in Gestational Diabetes Mellitus, Pregnancy Cholestasis, and the Coexistence of Both Diseases? International Journal of Environmental Research and Public Health. 2021; 18(23):12555. https://doi.org/10.3390/ijerph182312555
Chicago/Turabian StyleGüvey, Huri, Samettin Çelik, Canan Soyer Çalışkan, Zehra Yılmaz, Merve Yılmaz, Özlem Erten, and Andrea Tinelli. 2021. "How Do Serum Zonulin Levels Change in Gestational Diabetes Mellitus, Pregnancy Cholestasis, and the Coexistence of Both Diseases?" International Journal of Environmental Research and Public Health 18, no. 23: 12555. https://doi.org/10.3390/ijerph182312555
APA StyleGüvey, H., Çelik, S., Çalışkan, C. S., Yılmaz, Z., Yılmaz, M., Erten, Ö., & Tinelli, A. (2021). How Do Serum Zonulin Levels Change in Gestational Diabetes Mellitus, Pregnancy Cholestasis, and the Coexistence of Both Diseases? International Journal of Environmental Research and Public Health, 18(23), 12555. https://doi.org/10.3390/ijerph182312555