Health-Related Content of TV and Radio Advertising of Dietary Supplements—Analysis of Legal Aspects after Introduction of Self-Regulation for Advertising of These Products in Poland
Abstract
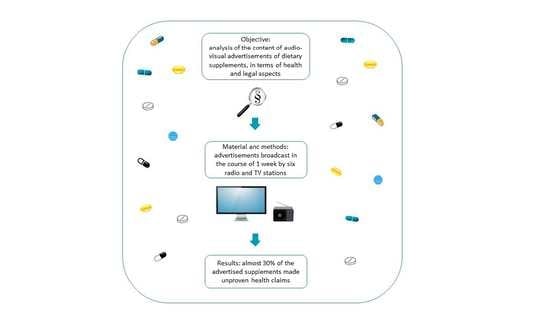
:1. Introduction
2. Material and Methods
- words and phrases which suggested that dietary supplements prevent or treat diseases or refer to such properties; or that were misleading as to the characteristics of the product and its nature; that attributed effects or properties which product does not possess; and that are unreliable (which is in breach of article 7 Regulation (EU) No. 1169/2011 of the European Parliament and of the Council as well as of the broadcasting agreement).
- health claims were not compliant with the legal registry of such claims (which is in breach of the Commission Regulation (EU) No. 432/2012), false information that guaranteed alleviation of health ailments, improved looks or mood; given that dietary supplement efficacy is not controlled by clinical studies, the producer may not promise that any effect on the consumer will be achieved (which is in breach of Regulation (EU) No. 1169/2011, Regulation (EC) No 1924/2006 and of the broadcasting agreement).
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Directive 2002/46/EC of the European Parliament and of the Council of 10 June 2002 on the approximation of the laws of the Member States relating to food supplements. Off. J. Eur. Communities 2002, L 183, 51–57.
- Makowska, M.; Jasiński, Ł. A discussion of the unresolved 2016/17 plans for regulating the Polish dietary supplements market. Health Policy 2019, 123, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Petroczi, A.; Taylor, G.; Naughton, D.P. Mission impossible? Regulatory and enforcement issues to ensure safety of dietary supplements. Food Chem. Toxicol. 2011, 49, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the provision of food information to consumers. Off. J. Eur. Union 2011, L 304, 18–63.
- Ronis, M.J.J.; Pedersen, K.B.; Watt, J. Adverse effects of nutraceuticals and dietary supplements. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 583–601. [Google Scholar] [CrossRef] [PubMed]
- Kennett, G. Time for change: Stepping up the FDA’s regulation of dietary supplements to promote consumer safety and awareness. J. Law Health 2019, 33, 47–78. [Google Scholar]
- Starr, R.R. Too little, too late: Ineffective regulation of dietary supplements in the United States. Am. J. Public Health 2015, 105, 478–485. [Google Scholar] [CrossRef]
- National Register of Dietary Supplements. Available online: https://powiadomienia.gis.gov.pl/ (accessed on 5 February 2022).
- Dietary Supplement Use Reaches All-Time High: Available-for-Purchase Consumer Survey Reaffirms the Vital Role Supplementation Plays in the Lives of most Americans. 2019. Available online: https://www.crnusa.org/newsroom/dietary-supplement-use-reaches-all-time-high-available-purchase-consumer-survey-reaffirms (accessed on 30 March 2022).
- Wawryk-Gawda, B.; Budzyńska, M.; Lis-Sochocka, P.; Chylińska-Wrzos, M.; Zarobkiewicz, B.; Jodłowska-Jędrych, B. Dietary supplements—Consumer assessment based on questionnaire survey. Przegl. Epidemiol. 2018, 72, 93–103. [Google Scholar]
- Waśkiewicz, A.; Sygnowska, E.; Broda, G.; Chwojnowska, Z. The use of vitamin supplements among adults in Warsaw: Is there any nutritional benefit? Rocz. Panstw. Zakl. Hig. 2014, 65, 119–126. [Google Scholar]
- Suliga, K.; Grzelak, T.; Grupińska, J.; Pelczyńska, M.; Sperling, M.; Czyżewska, K. Evaluation of using dietary supplements among polish adult people below and over 60 years of age. J. Med. Sci. 2017, 86, 213–219. [Google Scholar] [CrossRef] [Green Version]
- Sicińska, E.; Pietruszka, B.; Januszko, O.; Kałuza, J. Different socio-demographic and lifestyle factors can determine the dietary supplement use in children and adolescents in Central-Eastern Poland. Nutrients 2019, 11, 658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwingshackl, L.; Boeing, H.; Stelmach-Mardas, M.; Gottschald, M.; Dietrich, S.; Hoffmann, G.; Chaimani, A. Dietary supplements and risk of cause-specific death, cardiovascular disease, and cancer: A systematic review and meta-analysis of primary prevention trials. Adv. Nutr. 2017, 8, 27–39. [Google Scholar] [CrossRef]
- Chief Sanitary Inspectorate. Stan Sanitarny Kraju 2007-2013 (National Sanitary Status Report 2007–2013). Available online: https://www.gis.gov.pl (accessed on 2 April 2020).
- Polish Supreme Audit Office. On the Authorization of Dietary Supplements. 2017. Available online: https://www.nik.gov.pl/plik/id,13031,vp,15443.pdf. (accessed on 5 April 2020).
- Perelló-Oliver, S.; García-Arranz, A. Endorsers’ presence in regulation and endorsements in dietary supplements’ advertising on Spanish radio. Health Policy 2020, 124, 902–908. [Google Scholar]
- Koo, K.; Aro, T.; Matlaga, B.R. Buyer beware: Evidence-based evaluation of dietary supplements for nephrolithiasis. J. Endourol. 2020, 34, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Report of the National Broadcasting Council. Broadcasting Advertisements of Health Products and Drugs on Television Programs. Available online: http://www.krrit.gov.pl/Data/Files/_public/Portals/0/komunikaty/leki-i-suplementy/2015_emisja-przekazow-handlowych-produktow-zdrowotnych-lekow-w-programach-telewizyjnych.pdf (accessed on 12 December 2020).
- Wierzejska, R. Whether the advertisement of dietary supplements is objective source of data about their impact on health? Analysis of broadcasting advertisements in the terms of the food law. Wiadomości Lek. 2016, 69, 14–18. [Google Scholar]
- Report of the Advertising Ethics Committee. Available online: https://www.wirtualnemedia.pl/artykul/w-ub-r-ponad-dwa-razy-mniej-skarg-do-rady-reklamy-sporo-dotyczylo-kampanii-suplementow-diety (accessed on 15 June 2021).
- Genoscope. Available online: https://genoscope.pl/ (accessed on 25 March 2020).
- Adams, K.K.; Baker, W.L.; Sobieraj, D.M. Myth busters: Dietary supplements and COVID-19. Ann. Pharmacother. 2020, 54, 820–826. [Google Scholar] [CrossRef]
- Sirico, F.; Miressi, S.; Castaldo, C.; Spera, R.; Montagnani, S.; Di Meglio, F.; Nurzynska, D. Habits and beliefs related to food supplements: Results of a survey among Italian students of different education fields and levels. PLoS ONE 2018, 13, e0191424. [Google Scholar] [CrossRef]
- Lee, A.; Vásquez, L.J.; Wong, W.C.; Shin, J. Evaluation of dietary supplement advertisements in popular Spanish, Chinese, and Korean media outlets: A cross sectional study. BMC Nutr. 2015, 1, 43. [Google Scholar] [CrossRef] [Green Version]
- Shiragami, M. Problems with advertisements for health foods. Yakugaku Zasshi 2018, 138, 1523–1530. [Google Scholar] [CrossRef] [Green Version]
- Wierzejska, R.; Jarosz, M.; Siuba, M.; Rambuszek, M. Assessing patients’ attitude towards dietary supplements. Rocz. Panstw. Zakl. Hig. 2014, 65, 317–323. [Google Scholar]
- Karbownik, M.S.; Paul, E.; Nowicka, M.; Nowicka, Z.; Kowalczyk, R.P.; Kowalczyk, E.; Pietras, T. Knowledge about dietary supplements and trust in advertising them: Development and validation of the questionnaires and preliminary results of the association between the constructs. PLoS ONE 2019, 14, e0218398. [Google Scholar]
- White, C.M. Dietary supplements pose real dangers to patients. Ann. Pharmacother. 2020, 54, 815–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Broadcasting Council (Krajowa Rada Radiofonii i Telewizji). Available online: http://www.krrit.gov.pl/Data/Files/_public/Portals/0/samoregulacja-nadawcow/porozumienie-nadawcow---skan.pdf (accessed on 6 April 2020).
- Information about the Radio Auditorium in Poland in 2019. Available online: http://www.krrit.gov.pl/Data/Files/_public/Portals/0/Nadawcy/monitoring/rynek_radiowy-2019.pdf (accessed on 3 March 2020).
- Information on the Television Audience in Poland in 2019. Available online: http://www.krrit.gov.pl/Data/Files/_public/Portals/0/kontrola/program/tv/kwartalne/rynek-telewizyjny-w-2019-r.pdf (accessed on 3 March 2020).
- Act of 25 August 2006 on food and nutrition safety. Pol. J. Laws 2006, 171, 14365–14378.
- Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on nutrition and health claims made on foods. Off. J. Eur. Union 2006, L 404/9, 1–17. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32006R1924&from=en (accessed on 9 December 2021).
- Commission Regulation (EU) No 432/2012 of 16 May 2012 establishing a list of permitted health claims made on foods, other than those referring to the reduction of disease risk and to children’s development and health. Off. J. Eur. Union 2012, L 136, 1–40. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2012:136:0001:0040:en:PDF (accessed on 15 December 2021).
- Directive 2006/114/EC of the European Parliament and of the Council of 12 December 2006 concerning misleading and comparative advertising. Off. J. Eur. Union 2006, L 376, 1–7. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32006L0114 (accessed on 3 January 2022).
- Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. Off. J. Eur. Communities 2002, L 031, 1–24. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32002R0178&from=EN (accessed on 12 January 2022).
- Judgment of the Court (Fifth Chamber) of 30 November 1983. Case 227/82. Available online: https://eur-lex.europa.eu/legal-content/PL/TXT/?uri=CELEX%3A61982CJ0227 (accessed on 15 May 2020).
- Płudowski, P.; Ducki, C.; Konstantynowicz, J.; Jaworski, M. Vitamin D status in Poland. Pol. Arch. Med. Wewn. 2016, 126, 530–539. [Google Scholar] [CrossRef] [Green Version]
- Wierzejska, R.; Jarosz, M.; Sawicki, W.; Bachanek, M.; Siuba-Strzelińska, M. Vitamin D concentration in maternal and umbilical cord blood by season. Int. J. Environ. Res. Public Health 2017, 14, 1121. [Google Scholar] [CrossRef] [Green Version]
- Mirosz, P. Nutrition and health claims—Progress in work on nutrient profiles and Pending list. Hygeia Public Health 2017, 52, 21–25. [Google Scholar]
- European Food and Safety Authority (EFSA). Available online: http://registerofquestions.efsa.europa.eu/roqFrontend/wicket/page?4 (accessed on 12 April 2020).
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the Substantiation of Health Claims Related to Various Food(S)/Food Constituent(S) and Protection of Cells from Premature Aging, Antioxidant Activity, Antioxidant Content and Antioxidant Properties, and Protection of DNA, Proteins and Lipids from Oxidative Damage Pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2010, 8, 1489. Available online: https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2010.1489 (accessed on 14 April 2020).
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the Substantiation of Health Claims Related to Equisetum arvense L. and Invigoration of the Body (ID 2437), Maintenance of Skin (ID 2438), Maintenance of Hair (ID 2438), Maintenance of Bone (ID 2439), and Maintenance or Achievement of a Normal Body Weight (ID 2783) Pursuant to Article 13 of Regulation (EC) No 1924/2006. EFSA J. 2009, 7, 1289. Available online: https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2009.1289 (accessed on 14 April 2020).
- Chung, E.P.; Hwang, H.J.; Kim, M.K. Evaluation of non-English dietary supplement advertisement in an ethnic minority community in America. Pub. Health Nutr. 2007, 10, 834–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crawford, C.; Saldanha, L.; Costello, R.; Deuster, P.A. Dietary supplements for musculoskeletal pain: Science versus claims. J. Spec. Oper. Med. 2018, 18, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, R.B.; Hellberg, R.S. Shark cartilage supplement labeling practices and compliance with U.S. regulations. J. Diet. Suppl. 2019, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Crawford, C.; Boyd, C.; Avula, B.; Wang, Y.H.; Khan, I.A.; Deuster, P.A. A public health issue: Dietary supplements promoted for brain health and cognitive performance. J. Altern. Complement. Med. 2020, 26, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Okuhara, T.; Yokota, R.; Shirabe, R.; Iye, R.; Okada, H.; Kiuchi, T.; Chiba, T.; Akamatsu, R. Japanese newspaper advertisements for dietary supplements before and after COVID-19: A content analysis. BMJ Open 2021, 11, e050898. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, D.; Schwartz, J. Stopping deceptive health claims: The need for a private right of action under federal law. Am. J. Law Med. 2016, 41, 53–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanna, L.A.; Hughes, C.M. Public’s views on making decisions about over-the-counter medication and their attitudes towards evidence of effectiveness: A cross-sectional questionnaire study. Patient Educ. Couns. 2011, 83, 345–351. [Google Scholar]
| Purpose | No. of Dietary Supplements | Trade Name | Active Ingredients |
|---|---|---|---|
| Acti Vita-miner D3 | Vitamins (E, B1, B2, B6, B12, C, D, biotin, niacin, pantothenic acid, folic acid), minerals (iron, zinc, iodine), Tagetes erecta extract | ||
| Acti Vita-miner senior D3 | Vitamins (E, B1, B2, B6, B12, C, D, biotin, niacin, pantothenic acid, folic acid), minerals (iron, zinc, iodine, selenium), Tagetes erecta extract | ||
| Ascorvita Max | Vitamins (C, D), zinc | ||
| Blu Kid | Vitamin C, zinc, Sambucus nigra juice and extract, Citrus aurantium extract | ||
| Estabiom Baby | Vitamin D, Lactobacillus rhamnosus, Bifidobacterium breve, fructooligosaccharides | ||
| Immunity | 11 | Gripovita | Vitamin C, zinc, Sambucus nigra extract, Hibiscus splendens extract, Ribes nigrum dry concentrate, Rubus idaeus dry concentrate |
| Molekin D3 | Vitamin D | ||
| Pelavo Multi | Vitamin C, honey, Tilia cordata extract, Pelargonium sidoides extract, Rubus idaeus juice | ||
| Preventic D3 | Vitamin D, shark liver oil, Allium sativum oil | ||
| Rutinacea Junior | Vitamin C, rutin, Ribes nigrum concentrate, Rosa canina extract, Rubus idaeus juice | ||
| Rutinacea Max D3 | Vitamin C, minerals (zinc, selenium), rutozide, citrus bioflavonoids | ||
| Atresan | Glucosamine, methylsulfonylmethane, Zingiber officinale extract, vitamin C, copper | ||
| Collaflex | Vitamin C, collagen, chondroitin, hyaluronic acid, inulin | ||
| D-Vitum | Vitamin D, Linum usitatissimum oil | ||
| Joints, bones | 8 | D-Vitum forte K2 | Vitamins (D, K), Linum usitatissimum oil |
| Molekin D3+K2 | Vitamins (D, K) | ||
| Molekin Osteo | Vitamins (D, K), calcium | ||
| Vitrum 1250 Calcium | Vitamin D, calcium | ||
| 4Flex | Vitamin C, collagen | ||
| Climea forte | Vitamins (D, E, B6, folic acid), calcium, Glycine Willd extract, Humulus lupulus extract, Linum usitatissimum powder | ||
| Menopause | 3 | Duo-Fem | Vitamins (C, D, B6, E), minerals (magnesium, zinc, chrome), Trifolium pratense extract, Melissa officinalis extract, Humulus lupulus extract |
| Femitonina | Melatonin, Glycine Willd extract, Trifolium pratense extract, Humulus lupulus extract | ||
| Dieting | 3 | Liporedium | Cola nitida extract, Capsicum annuum extract, Ilex paraguariensis extract, Garcinia cambogia extract |
| Slimbel-3 fazy | Vitamins (C, A, E, niacin, pantothenic acid, B1, B2, B6, folic acid, biotin, B12), minerals (magnesium, iron, manganese, copper, zinc), L-carnitin, Sambucus nigra juice, Smilax officinalis extract, Citrus limon juice, Foeniculum vulgare extract, Rosmarinus officinalis extract, Coenzyme Q10, Fucus vesiculosus extract, Camellia sinensis (green tea) extract, Coffea arabica extract, Sambucus nigra concentrate, Ananas Mill. concentrate, Melissa officinalis extract, Tilia cordata extract, Sambucus nigra extract, Smilax officinalis extract, Verbena officinalis extract | ||
| Term Line | L-thyroxine, L-carnitin, chrome, citrus fruit extract, Camellia sinensis (green tea) extract, caffeine, Paullinia cupana extract, Coffea arabica extract, Zingiber officinale extract, Capsicum annuum extract, Piper nigrum extract | ||
| Gold-Luteina | Vitamins (E, A), zinc, fish oil, lutein and zeaxantin from Tagetes erecta | ||
| Eyes | 3 | Nutrof Total | Vitamins (C, E, D), minerals (zinc, copper, selenium), lutein, zeaxantin, fish oil, Crocus sativus oil, Vitis vinifera extract |
| Vizik max | Vitamins (C, B2, B6, B12, E, A), minerals (selenium, zinc), Tagetes erecta extract | ||
| Apetizer Senior | Vitamins (B1, B2, B6, B12, folic acid, biotin, niacin, pantothenic acid), Ribes nigrum concentrate, Pimpinella anisum extract, Cichorium intybus extract, Mentha piperita extract, Anethum graveolens extract, Citrus paradisi extract | ||
| Appetite, digestion | 3 | Travisto | Cynara scolymus extract, Curcuma longa extract, Mentha piperita extract, Anethum graveolens extract |
| Verdin Complexx | Rosmarinus officinalis powder, Rosmarinus officinalis extract, Cynara scolymus powder, Cynara scolymus extract, Curcuma longa extract, Mentha piperita oil | ||
| Novanoc | Vitamin B6, melatonin, Passiflora caerulea extract, Melissa officinalis extract, Eschscholzia californica extract, Arthrospira platensis extract | ||
| Nerves, sleep | 3 | Positivum | Melissa officinalis extract, Humulus lupulus extract, Crocus sativus extract |
| Valerin Sen | Vitamin B6, magnesium, Melissa officinalis extract, Humulus lupulus extract, L-theanine, L-tryptophan, Gamma-aminobutyric acid (GABA), Crocus sativus extract | ||
| Hair | 2 | Skrzypolen | Vitamin (D, A, E, biotin), minerals (selenium, zinc), taurine, L-methionine, collagen, Equisetum arvense extract, Linum usitatissimum extract, Urtica dioica extract |
| Skrzypovita | Vitamins (C, B1, B2, B6, A, E, biotin, pantothenic acid), minerals (zinc, copper), para-aminobenzoic acid (PABA), collagen, hyaluronic acid, Equisetum arvense extract, Urtica dioica extract | ||
| Liver | 1 | Hepaslimin | L-ornithine, choline, Ilex paraguariensis extract, Cynara scolymus extract, Curcuma longa extract, Cichorium intybus extract |
| Memory | 1 | Bilomag Plus | Vitamins (B6, pantothenic acid), magnesium, Ginkgo biloba extract, lecithin |
| Heart | 1 | Gold Omega 3 D3 + K2 | Vitamins (D, K, E), fish oil |
| Urinary incontinence | 1 | Feminost | Vitamins (D, B12), Cucurbita pepo extract, Vaccinium oxycoccos juice, Glycine clandestina extract, Urtica dioica extract |
| Throat | 1 | Fiorda | Lactoferrin, Cetraria islandica extract, Rosa canina extract, Rubus ideaus concentrate, Sambucus nigra juice |
| Pregnancy | 1 | Profolium | 5-methyltetrahydrofolic acid |
| General support for the normal functioning of the body | 4 | Vitotal | Vitamins (C, E, A, B1, B2, B6, B12, D, K, niacin, pantothenic acid, biotin), minerals (zinc, iron, magnesium, manganese, copper, iodine, molybdenum, chrome, selenium), collagen, L-cysteine, L-methionine, lycopene, lutein, Equisetum arvense extract, Vitis vinifera extract |
| Solevitum D3 | Vitamin D | ||
| NeoMag forte D3 | Vitamins (D, B6), magnesium | ||
| D-Vitum DHA | Vitamin D, Schizochytrium sp. oil |
| ACTION OF DIETARY SUPPLEMENTS ACCORDING TO ADVERTISERS |
|---|
| Type of Claim |
| Part A. Unreliable guaranteed action claims (non-compliant with Regulation (EU) No. 1169/2011, Regulation (EC) No. 1924/2006 and with the agreement) |
| 1.12 weeks and this is the effect (images of sporty people, indicating a well-functioning osteoarticular system) 2.I use (name of the product) …and I no longer fear hot flashes, mood swings, night sweats and excessive weight 3.(name of the product) … and I remember everything. I use it so my memory will never fail me 4.The kilograms disappear forever, effective weight loss 5.For losing weight—verified and effective 6.With … (name of the product) I no longer worry about cellulite 7.You will calm down, you will get a good night’s sleep 8.(name of the product) … means healthy liver and additionally healthy weight 9.(name of the product) … helped me; problem with urinary continence—not my problem any more 10.(name of the product) … equals a good night’s sleep 11.Your hair becomes thicker, more shiny and healthier |
| Part B. Support claims |
| 1.Supports immunity 2.A way to boost your immunity 3.Allows you to take care of your immunity 4.For problems with appetite,promotes appetite and facilitates digestion 5.Aids digestion and reduction of excess gasses, supports stomach, liver and gut function 6.Supports collagen production 7.Good for your joints 8.For strong and healthy bones 9.Protects your throat 10.Takes care of the microbiome 11.Relieves menopausal symptoms 12.Complete supports your sight 13.Supports proper vision 14.Helps me keep my weight 15.Helps you fall asleep faster 16.Supports uninterrupted sleep and gives you energy 17.Helps deal with stress 18.A way to lose weight 19.Offers complex support |
| ACTION OF SUPPLEMENT INGREDIENTS ACCORDING TO ADVERTISERS |
| Type of Claim |
| Part C. Unreliable guaranteed action claims (non-compliant with Regulation (EU) No. 1169/2011, Regulation (EC) No. 1924/2006 and with the agreement) |
| 1.Extract of turmeric prevents accumulation of fats 2.Herbal extracts for improved digestion, which eliminate the feeling of heaviness 3.Active ingredients maintain proper muscle tone, including the muscles responsible for urinary continence |
| Part D. Unreliable support claims |
| 1.Zinc has beneficial effect on tired eyes (non-compliant with the Commission Regulation (EU) No. 432/2012) 2.Extract of Cola nitida intensively supports fat burning, helps effective reduction of body weight (non-compliant with Regulation (EC) No. 1924/2006) |
| Part E. Other support Claims |
| 1.Vitamin D contributes to normal function of the immune system 2.Vitamin D contributes to maintenance of normal bones, teeth, and muscle function 3.Vitamin D is essential for healthy bones 4.Vitamin D contributes to normal absorption of calcium 5.Vitamin Dcontributes to healthy growth/development of children 6.Vitamin D is vital for immunity 7.Vitamin D supports immunity 8.Vitamin C contributes to normal collagen formation for the normal function of skin and cartilage 9.Vitamin C contributes to the normal function of the immune system 10.Vitamin A contributes to the normal function of the immune system 11.Vitamin A contributes to the maintenance of normal skin 12.Vitamin A supports immunity 13.Vitamin K2 for healthy bones 14.Biotin contributes to the maintenance of normal hair and skin 15.Biotin contributes to normal psychological function 16.Zinc contributes to the maintenance of normal vision 17.Magnesium contributes to normal functioning of the nervous system 18.Calcium contributes to normal bone, teeth, and muscle function 19.Omega-3 fatty acids contribute to normal cardiac function 20.DHA contributes to normal brain function in children 21.Melatonin helps you fall asleep 22.Garlic supports anti-oxidative ability of the body and its natural defense mechanism 23.Garlic supports defense mechanisms and helps maintain healthy respiratory system 24.Extract of rosa canina supports immunity 25.Extract of peppermint helps digestion and proper function of the digestive tract, and maintain healthy stomach 26.Extract of clover helps alleviate menopausal symptoms 27.Extract of humulus strobiles helps alleviate menopausal symptoms 28.Extract of humulus strobiles contributes to peaceful sleep 29.Cayenne pepper helps reduce body weight 30.Yerba mate helps maintain proper body weight 31.Extract of artichoke leaves supports detoxication, stimulates digestive acid secretion, and helps maintain healthy liver; assures intestine comfort 32.Extract of dill flowers supports digestion, elimination of excess gas, and helps proper digestion of fats/lipids 33.Extract of turmeric helps you maintain normal liver function 34.Extract of saffron crocus increases emotional balance 35.Extract of grape seedshelps maintain proper body weight and reduce cellulite 36.Artichoke, rosemary, turmeric help digestion and digestive comfort 37.Field horsetail helps you keep healthy hair, skin, and nails 38.Flax supports weight control 39.Amino acids are the building blocks of keratin, naturally found in hair and eyelashes 40.Ingredients help maintain concentration and mental agility 41.Ingredients soothe hot flashes and irritability, promote peaceful sleep and youthful appearance |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wierzejska, R.E.; Wiosetek-Reske, A.; Siuba-Strzelińska, M.; Wojda, B. Health-Related Content of TV and Radio Advertising of Dietary Supplements—Analysis of Legal Aspects after Introduction of Self-Regulation for Advertising of These Products in Poland. Int. J. Environ. Res. Public Health 2022, 19, 8037. https://doi.org/10.3390/ijerph19138037
Wierzejska RE, Wiosetek-Reske A, Siuba-Strzelińska M, Wojda B. Health-Related Content of TV and Radio Advertising of Dietary Supplements—Analysis of Legal Aspects after Introduction of Self-Regulation for Advertising of These Products in Poland. International Journal of Environmental Research and Public Health. 2022; 19(13):8037. https://doi.org/10.3390/ijerph19138037
Chicago/Turabian StyleWierzejska, Regina Ewa, Agnieszka Wiosetek-Reske, Magdalena Siuba-Strzelińska, and Barbara Wojda. 2022. "Health-Related Content of TV and Radio Advertising of Dietary Supplements—Analysis of Legal Aspects after Introduction of Self-Regulation for Advertising of These Products in Poland" International Journal of Environmental Research and Public Health 19, no. 13: 8037. https://doi.org/10.3390/ijerph19138037
APA StyleWierzejska, R. E., Wiosetek-Reske, A., Siuba-Strzelińska, M., & Wojda, B. (2022). Health-Related Content of TV and Radio Advertising of Dietary Supplements—Analysis of Legal Aspects after Introduction of Self-Regulation for Advertising of These Products in Poland. International Journal of Environmental Research and Public Health, 19(13), 8037. https://doi.org/10.3390/ijerph19138037