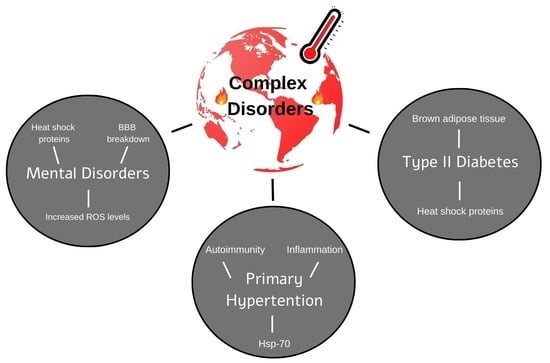
The Effect of Global Warming on Complex Disorders (Mental Disorders, Primary Hypertension, and Type 2 Diabetes)
Abstract
:1. Introduction
2. Global Warming and Mental Disorders
3. Global Warming and Mental Disorders—Potential Mechanisms
3.1. The Heat Shock Proteins (HSPs) System
3.2. The Link between Heat Stress (HS) and Oxidative Stress
3.3. Heat Stress and the Blood-Brain Barrier (BBB)
4. Global Warming and Primary Hypertension
5. Global Warming and Primary Hypertension—Potential Mechanisms
5.1. Hyperosmolarity
5.2. Autoimmunity, Inflammation, Sodium Excretion and HSP70
6. Global Warming and Type 2 Diabetes
7. Global Warming and Type 2 Diabetes—Potential Mechanisms
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ebi, K.L. Managing climate change risks is imperative for human health. Nat. Rev. Nephrol. 2022, 18, 74–75. [Google Scholar] [CrossRef]
- Vogel, M.M.; Zscheischler, J.; Wartenburger, R.; Dee, D.; Seneviratne, S.I. Concurrent 2018 Hot Extremes Across Northern Hemisphere Due to Human-Induced Climate Change. Earths Futur. 2019, 7, 692–703. [Google Scholar] [CrossRef] [PubMed]
- Craig, J. Complex diseases. Res. Appl. 2008, 1, 184. [Google Scholar]
- Bolton, D. What is Mental Disorder? An Essay in Philosophy, Science, and Values; Oxford University Press: Oxford, UK, 2008. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services, Substance Abuse and Mental Health Services Administration. Center for Behavioral Health Statistics and Quality Key Substance Use and Mental Health Indicators in the United States: Results from the 2020 National Survey on Drug Use and Health; HHS Publication: Rockville, MD, USA, 2021.
- Zhou, B.; Perel, P.; Mensah, G.A.; Ezzati, M. Global epidemiology, health burden and effective interventions for elevated blood pressure and hypertension. Nat. Rev. Cardiol. 2021, 18, 785–802. [Google Scholar] [CrossRef]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martin, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef]
- Kirkman, M.S.; Briscoe, V.J.; Clark, N.; Florez, H.; Haas, L.B.; Halter, J.B.; Huang, E.S.; Korytkowski, M.T.; Munshi, M.N.; Odegard, P.S.; et al. Diabetes in older adults: A consensus report. J. Am. Geriatr. Soc. 2012, 60, 2342–2356. [Google Scholar] [CrossRef]
- Einarson, T.R.; Acs, A.; Ludwig, C.; Panton, U.H. Prevalence of cardiovascular disease in type 2 diabetes: A systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc. Diabetol. 2018, 17, 83. [Google Scholar] [CrossRef] [Green Version]
- Obradovich, N.; Migliorini, R.; Paulus, M.P.; Rahwan, I. Empirical evidence of mental health risks posed by climate change. Proc. Natl. Acad. Sci. USA 2018, 115, 10953–10958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, Z.; Wang, Q.; Kan, H.; Chen, R.; Wang, W. Effects of ambient temperature on daily hospital admissions for mental disorders in Shanghai, China: A time-series analysis. Sci. Total Environ. 2017, 590–591, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Almendra, R.; Loureiro, A.; Silva, G.; Vasconcelos, J.; Santana, P. Short-term impacts of air temperature on hospitalizations for mental disorders in Lisbon. Sci. Total Environ. 2019, 647, 127–133. [Google Scholar] [CrossRef]
- Mullins, J.T.; White, C. Temperature and mental health: Evidence from the spectrum of mental health outcomes. J. Health Econ. 2019, 68, 102240. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.J.; Kim, L.; Joe, S.H.; Suh, K.Y. Effects of season and climate on the first manic episode of bipolar affective disorder in Korea. Psychiatry Res. 2002, 113, 151–159. [Google Scholar] [CrossRef]
- Chen, N.T.; Lin, P.H.; Guo, Y.L. Long-term exposure to high temperature associated with the incidence of major depressive disorder. Sci. Total Environ. 2019, 659, 1016–1020. [Google Scholar] [CrossRef]
- Ranson, M. Crime, weather, and climate change. J. Environ. Econ. Manag. 2014, 67, 274–302. [Google Scholar] [CrossRef]
- Krug, E.G.; Mercy, J.A.; Dahlberg, L.L.; Zwi, A.B. The world report on violence and health. Lancet 2002, 360, 1083–1088. [Google Scholar] [CrossRef] [Green Version]
- Palinkas, L.A.; Wong, M. Global climate change and mental health. Curr. Opin. Psychol. 2020, 32, 12–16. [Google Scholar] [CrossRef]
- Hayes, K.; Blashki, G.; Wiseman, J.; Burke, S.; Reifels, L. Climate change and mental health: Risks, impacts and priority actions. Int. J. Ment. Health Syst. 2018, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- Cianconi, P.; Betro, S.; Janiri, L. The Impact of Climate Change on Mental Health: A Systematic Descriptive Review. Front. Psychiatry 2020, 11, 74. [Google Scholar] [CrossRef]
- Berry, H.L.; Bowen, K.; Kjellstrom, T. Climate change and mental health: A causal pathways framework. Int. J. Public Health 2010, 55, 123–132. [Google Scholar] [CrossRef]
- Horowitz, M. Heat acclimation, epigenetics, and cytoprotection memory. Compr. Physiol. 2014, 4, 199–230. [Google Scholar] [CrossRef] [PubMed]
- Periard, J.D.; Racinais, S.; Sawka, M.N. Adaptations and mechanisms of human heat acclimation: Applications for competitive athletes and sports. Scand J. Med. Sci. Sports 2015, 25 (Suppl. 1), 20–38. [Google Scholar] [CrossRef] [PubMed]
- Domanico, S.Z.; DeNagel, D.C.; Dahlseid, J.N.; Green, J.M.; Pierce, S.K. Cloning of the gene encoding peptide-binding protein 74 shows that it is a new member of the heat shock protein 70 family. Mol. Cell Biol. 1993, 13, 3598–3610. [Google Scholar] [CrossRef]
- Giebel, L.B.; Dworniczak, B.P.; Bautz, E.K. Developmental regulation of a constitutively expressed mouse mRNA encoding a 72-kDa heat shock-like protein. Dev. Biol. 1988, 125, 200–207. [Google Scholar] [CrossRef]
- Amorim, F.T.; Fonseca, I.T.; Machado-Moreira, C.A.; Magalhaes Fde, C. Insights into the role of heat shock protein 72 to whole-body heat acclimation in humans. Temperature 2015, 2, 499–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akerfelt, M.; Morimoto, R.I.; Sistonen, L. Heat shock factors: Integrators of cell stress, development and lifespan. Nat. Rev. Mol. Cell Biol. 2010, 11, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Hung, J.J.; Cheng, T.J.; Chang, M.D.; Chen, K.D.; Huang, H.L.; Lai, Y.K. Involvement of heat shock elements and basal transcription elements in the differential induction of the 70-kDa heat shock protein and its cognate by cadmium chloride in 9L rat brain tumor cells. J. Cell Biochem. 1998, 71, 21–35. [Google Scholar] [CrossRef]
- Li, G.C.; Mivechi, N.F.; Weitzel, G. Heat shock proteins, thermotolerance, and their relevance to clinical hyperthermia. Int. J. Hyperth. 1995, 11, 459–488. [Google Scholar] [CrossRef]
- Raichle, M.E.; Gusnard, D.A. Appraising the brain’s energy budget. Proc. Natl. Acad. Sci. USA 2002, 99, 10237–10239. [Google Scholar] [CrossRef] [Green Version]
- Sokoloff, L. Energetics of functional activation in neural tissues. Neurochem. Res. 1999, 24, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Watts, M.E.; Pocock, R.; Claudianos, C. Brain Energy and Oxygen Metabolism: Emerging Role in Normal Function and Disease. Front. Mol. Neurosci. 2018, 11, 216. [Google Scholar] [CrossRef] [PubMed]
- Cadenas, E.; Davies, K.J. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol. Med. 2000, 29, 222–230. [Google Scholar] [CrossRef]
- Katschinski, D.M.; Boos, K.; Schindler, S.G.; Fandrey, J. Pivotal role of reactive oxygen species as intracellular mediators of hyperthermia-induced apoptosis. J. Biol. Chem. 2000, 275, 21094–21098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Orabi, N.F.; Rogers, C.B.; Edwards, H.G.; Schwartz, D.D. Heat-induced inhibition of superoxide dismutase and accumulation of reactive oxygen species leads to HT-22 neuronal cell death. J. Therm. Biol. 2011, 36, 49–56. [Google Scholar] [CrossRef]
- Freeman, M.L.; Spitz, D.R.; Meredith, M.J. Does heat shock enhance oxidative stress? Studies with ferrous and ferric iron. Radiat. Res. 1990, 124, 288–293. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Vida, S.; Durocher, M.; Ouarda, T.B.; Gosselin, P. Relationship between ambient temperature and humidity and visits to mental health emergency departments in Quebec. Psychiatr. Serv. 2012, 63, 1150–1153. [Google Scholar] [CrossRef] [PubMed]
- Giles, D.A.; Moreno-Fernandez, M.E.; Stankiewicz, T.E.; Graspeuntner, S.; Cappelletti, M.; Wu, D.; Mukherjee, R.; Chan, C.C.; Lawson, M.J.; Klarquist, J.; et al. Thermoneutral housing exacerbates nonalcoholic fatty liver disease in mice and allows for sex-independent disease modeling. Nat. Med. 2017, 23, 829–838. [Google Scholar] [CrossRef] [Green Version]
- Hanin, I. The Gulf War, stress and a leaky blood-brain barrier. Nat. Med. 1996, 2, 1307–1308. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.P.; Frey, B.N. Disruption in the Blood-Brain Barrier: The Missing Link between Brain and Body Inflammation in Bipolar Disorder? Neural Plast. 2015, 2015, 708306. [Google Scholar] [CrossRef]
- McEwen, B.S.; Wingfield, J.C. What is in a name? Integrating homeostasis, allostasis and stress. Horm. Behav. 2010, 57, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Elias, A.; Mrkonjic, S.; Jung, C.; Pardo-Pastor, C.; Vicente, R.; Valverde, M.A. The TRPV4 channel. Handb. Exp. Pharmacol. 2014, 222, 293–319. [Google Scholar] [CrossRef] [PubMed]
- Shibasaki, K.; Sugio, S.; Takao, K.; Yamanaka, A.; Miyakawa, T.; Tominaga, M.; Ishizaki, Y. TRPV4 activation at the physiological temperature is a critical determinant of neuronal excitability and behavior. Pflugers Arch. 2015, 467, 2495–2507. [Google Scholar] [CrossRef] [PubMed]
- Lipski, J.; Park, T.I.; Li, D.; Lee, S.C.; Trevarton, A.J.; Chung, K.K.; Freestone, P.S.; Bai, J.Z. Involvement of TRP-like channels in the acute ischemic response of hippocampal CA1 neurons in brain slices. Brain Res. 2006, 1077, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Benfenati, V.; Amiry-Moghaddam, M.; Caprini, M.; Mylonakou, M.N.; Rapisarda, C.; Ottersen, O.P.; Ferroni, S. Expression and functional characterization of transient receptor potential vanilloid-related channel 4 (TRPV4) in rat cortical astrocytes. Neuroscience 2007, 148, 876–892. [Google Scholar] [CrossRef] [PubMed]
- Konno, M.; Shirakawa, H.; Iida, S.; Sakimoto, S.; Matsutani, I.; Miyake, T.; Kageyama, K.; Nakagawa, T.; Shibasaki, K.; Kaneko, S. Stimulation of transient receptor potential vanilloid 4 channel suppresses abnormal activation of microglia induced by lipopolysaccharide. Glia 2012, 60, 761–770. [Google Scholar] [CrossRef]
- Hatano, N.; Suzuki, H.; Itoh, Y.; Muraki, K. TRPV4 partially participates in proliferation of human brain capillary endothelial cells. Life Sci. 2013, 92, 317–324. [Google Scholar] [CrossRef]
- Jie, P.; Hong, Z.; Tian, Y.; Li, Y.; Lin, L.; Zhou, L.; Du, Y.; Chen, L.; Chen, L. Activation of transient receptor potential vanilloid 4 induces apoptosis in hippocampus through downregulating PI3K/Akt and upregulating p38 MAPK signaling pathways. Cell Death Dis. 2015, 6, e1775. [Google Scholar] [CrossRef] [Green Version]
- Narita, K.; Sasamoto, S.; Koizumi, S.; Okazaki, S.; Nakamura, H.; Inoue, T.; Takeda, S. TRPV4 regulates the integrity of the blood-cerebrospinal fluid barrier and modulates transepithelial protein transport. FASEB J. 2015, 29, 2247–2259. [Google Scholar] [CrossRef]
- Halonen, J.I.; Zanobetti, A.; Sparrow, D.; Vokonas, P.S.; Schwartz, J. Relationship between outdoor temperature and blood pressure. Occup. Environ. Med. 2011, 68, 296–301. [Google Scholar] [CrossRef] [Green Version]
- Stotz, A.; Rapp, K.; Oksa, J.; Skelton, D.A.; Beyer, N.; Klenk, J.; Becker, C.; Lindemann, U. Effect of a brief heat exposure on blood pressure and physical performance of older women living in the community-a pilot-study. Int. J. Environ. Res. Public Health 2014, 11, 12623–12631. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Liu, F.; Ban, J.; Zhang, Y.; Li, T. Short-term effects of ambient temperature on blood pressure: A systematic review and meta-analysis. Cardiol. Plus 2017, 2, 18–25. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Powles, J.W. The effect of ambient temperature on blood pressure in a rural West African adult population: A cross-sectional study. Cardiovasc. J. Afr. 2010, 21, 17–20. [Google Scholar] [PubMed]
- Rodriguez-Iturbe, B.; Lanaspa, M.A.; Johnson, R.J. The role of autoimmune reactivity induced by heat shock protein 70 in the pathogenesis of essential hypertension. Br. J. Pharmacol. 2019, 176, 1829–1838. [Google Scholar] [CrossRef] [PubMed]
- Crandall, C.G.; Wilson, T.E. Human cardiovascular responses to passive heat stress. Compr. Physiol. 2015, 5, 17–43. [Google Scholar] [CrossRef] [Green Version]
- Pons, H.; Ferrebuz, A.; Quiroz, Y.; Romero-Vasquez, F.; Parra, G.; Johnson, R.J.; Rodriguez-Iturbe, B. Immune reactivity to heat shock protein 70 expressed in the kidney is cause of salt-sensitive hypertension. Am. J. Physiol. Renal. Physiol. 2013, 304, F289–F299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, Y.; Han, Y.; Guan, T.; Wang, X.; Xue, T.; Chen, Z.; Jiang, L.; Zhang, L.; Zheng, C.; Wang, Z.; et al. Clinical blood pressure responses to daily ambient temperature exposure in China: An analysis based on a representative nationwide population. Sci. Total Environ. 2020, 705, 135762. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kario, K.; Chia, Y.C.; Turana, Y.; Chen, C.H.; Buranakitjaroen, P.; Nailes, J.; Hoshide, S.; Siddique, S.; Sison, J.; et al. The influence of the ambient temperature on blood pressure and how it will affect the epidemiology of hypertension in Asia. J. Clin. Hypertens. 2020, 22, 438–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jehn, M.; Appel, L.J.; Sacks, F.M.; Miller, E.R., 3rd; DASH Collaborative Research Group. The effect of ambient temperature and barometric pressure on ambulatory blood pressure variability. Am. J. Hypertens. 2002, 15, 941–945. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Li, C.; Guo, Y.; Barnett, A.G.; Tong, S.; Phung, D.; Chu, C.; Dear, K.; Wang, X.; Huang, C. Environmental ambient temperature and blood pressure in adults: A systematic review and meta-analysis. Sci. Total Environ. 2017, 575, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.; Schneider, A. Cardiovascular risks of climate change. Nat. Rev. Cardiol. 2021, 18, 1–2. [Google Scholar] [CrossRef]
- Johnson, R.J.; Sanchez-Lozada, L.G.; Newman, L.S.; Lanaspa, M.A.; Diaz, H.F.; Lemery, J.; Rodriguez-Iturbe, B.; Tolan, D.R.; Butler-Dawson, J.; Sato, Y.; et al. Climate Change and the Kidney. Ann. Nutr. Metab. 2019, 74 (Suppl. 3), 38–44. [Google Scholar] [CrossRef] [PubMed]
- Glaser, J.; Lemery, J.; Rajagopalan, B.; Diaz, H.F.; Garcia-Trabanino, R.; Taduri, G.; Madero, M.; Amarasinghe, M.; Abraham, G.; Anutrakulchai, S.; et al. Climate Change and the Emergent Epidemic of CKD from Heat Stress in Rural Communities: The Case for Heat Stress Nephropathy. Clin. J. Am. Soc. Nephrol. 2016, 11, 1472–1483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ephraim, R.K.D.; Asamoah, C.A.; Abaka-Yawson, A.; Kwadzokpui, P.K.; Adusei, S. Climate change causes changes in biochemical markers of kidney disease. BMC Nephrol. 2020, 21, 542. [Google Scholar] [CrossRef] [PubMed]
- Katori, M.; Majima, M. A missing link between a high salt intake and blood pressure increase. J. Pharmacol. Sci. 2006, 100, 370–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Iturbe, B.; Pons, H.; Johnson, R.J. Role of the Immune System in Hypertension. Physiol. Rev. 2017, 97, 1127–1164. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Iturbe, B.; Pons, H.; Quiroz, Y.; Johnson, R.J. The immunological basis of hypertension. Am. J. Hypertens. 2014, 27, 1327–1337. [Google Scholar] [CrossRef] [Green Version]
- Janeway, C.A., Jr.; Travers, P.; Walport, M.; Capra, D.J. Immunobiology: The Immune System in Health and Disease, 5th ed.; Garland Science: New York, NY, USA, 2001. [Google Scholar]
- Seposo, X.T.; Dang, T.N.; Honda, Y. How Does Ambient Air Temperature Affect Diabetes Mortality in Tropical Cities? Int. J. Environ. Res. Public Health 2017, 14, 385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajat, S.; Haines, A.; Sarran, C.; Sharma, A.; Bates, C.; Fleming, L.E. The effect of ambient temperature on type-2-diabetes: Case-crossover analysis of 4+ million GP consultations across England. Environ. Health 2017, 16, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, R.; Zhao, Q.; Coelho, M.; Saldiva, P.H.N.; Zoungas, S.; Huxley, R.R.; Abramson, M.J.; Guo, Y.; Li, S. Association between Heat Exposure and Hospitalization for Diabetes in Brazil during 2000-2015: A Nationwide Case-Crossover Study. Environ. Health Perspect. 2019, 127, 117005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zilbermint, M. Diabetes and climate change. J. Community Hosp. Intern. Med. Perspect. 2020, 10, 409–412. [Google Scholar] [CrossRef]
- Auliyani, D.; Wahyuningrum, N.; Supangat, A.B.; Basuki, T.M. The bidirectional interaction between climate change and type 2 diabetes burden. In Proceedings of the IOP Conference Series: Earth and Environmental Science, the 7th International Conference on Climate Change, Virtual, 17–18 November 2021. [Google Scholar]
- Alemi, H.; Khaloo, P.; Rabizadeh, S.; Mansournia, M.A.; Mirmiranpour, H.; Salehi, S.S.; Esteghamati, A.; Nakhjavani, M. Association of extracellular heat shock protein 70 and insulin resistance in type 2 diabetes; independent of obesity and C-reactive protein. Cell Stress Chaperones 2019, 24, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Hooper, P.L.; Hooper, P.L. Inflammation, heat shock proteins, and type 2 diabetes. Cell Stress Chaperones 2009, 14, 113–115. [Google Scholar] [CrossRef] [Green Version]
- Zilaee, M.; Shirali, S. Heat Shock Proteins and Diabetes. Can. J. Diabetes 2016, 40, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Blauw, L.L.; Aziz, N.A.; Tannemaat, M.R.; Blauw, C.A.; de Craen, A.J.; Pijl, H.; Rensen, P.C.N. Diabetes incidence and glucose intolerance prevalence increase with higher outdoor temperature. BMJ Open Diabetes Res. Care 2017, 5, e000317. [Google Scholar] [CrossRef] [PubMed]
- Koranyi, L.; Hegedus, E.; Peterfal, E.; Kurucz, I. The role of hsp60 and hsp70 kDa heat shock protein families in different types of diabetes mellitus. Orvosi Hetil. 2004, 145, 467–472. [Google Scholar]
- Krause, M.; Ludwig, M.S.; Heck, T.G.; Takahashi, H.K. Heat shock proteins and heat therapy for type 2 diabetes: Pros and cons. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Li, S.; Guo, S.; Zhao, Q.; Abramson, M.J.; Li, S.; Guo, Y. Environmental temperature and human epigenetic modifications: A systematic review. Environ. Pollut. 2020, 259, 113840. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Natur, S.; Damri, O.; Agam, G. The Effect of Global Warming on Complex Disorders (Mental Disorders, Primary Hypertension, and Type 2 Diabetes). Int. J. Environ. Res. Public Health 2022, 19, 9398. https://doi.org/10.3390/ijerph19159398
Natur S, Damri O, Agam G. The Effect of Global Warming on Complex Disorders (Mental Disorders, Primary Hypertension, and Type 2 Diabetes). International Journal of Environmental Research and Public Health. 2022; 19(15):9398. https://doi.org/10.3390/ijerph19159398
Chicago/Turabian StyleNatur, Sarya, Odeya Damri, and Galila Agam. 2022. "The Effect of Global Warming on Complex Disorders (Mental Disorders, Primary Hypertension, and Type 2 Diabetes)" International Journal of Environmental Research and Public Health 19, no. 15: 9398. https://doi.org/10.3390/ijerph19159398
APA StyleNatur, S., Damri, O., & Agam, G. (2022). The Effect of Global Warming on Complex Disorders (Mental Disorders, Primary Hypertension, and Type 2 Diabetes). International Journal of Environmental Research and Public Health, 19(15), 9398. https://doi.org/10.3390/ijerph19159398