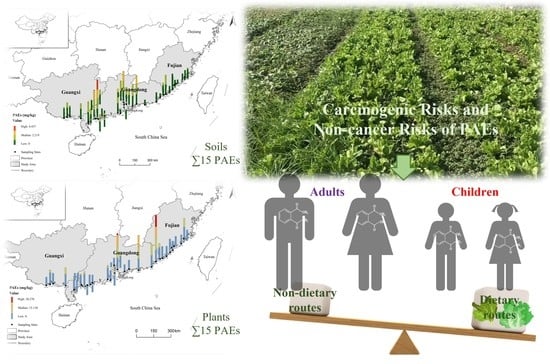
Characteristics and Health Risks of Phthalate Ester Contamination in Soil and Plants in Coastal Areas of South China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Information
2.2. Chemicals and Reagents
2.3. Sample Extraction and Purification
2.4. Instrumental Analysis
2.5. Quality Assurance and Quality Control
2.6. Statistical Analysis
2.7. Health Risk Assessments
3. Results and Discussion
3.1. Overall Characteristics of PAE Contamination in Soil
3.2. Regional Differences in PAE Contamination
3.3. Concentration and Distribution of PAEs in Plants
| Location | Plant Types | DMP | DEP | DnBP | BBP | DEHP | DnOP | Σ6 PAEs | Σ15 PAEs | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Pearl River Delta | Vegetables | ND–0.69 | ND–0.084 | ND–2.03 | ND–9.7 | ND–9.3 | ND–0.47 | 0.15–11.2 | - | [54] |
| southern and northern provinces in China | Benincasa hispida | NA | NA | NA | NA | 2.6–75.5 | NA | - | - | [60] |
| Netherlands | Vegetation | NA | NA | ND | NA | 0.0418b | NA | - | - | [51] |
| Dalian of Northeast China | Plant | NA | NA | 1.33b | NA | 2.84b | NA | 2.44–21.8 | - | [27] |
| Suburban plastic film greenhouses | Vegetables | ND–0.15 | ND–0.35 | 0.13–1.81 | ND–0.09 | 0.12–5.82 | ND–1.31 | 0.51–7.16 | - | [19] |
| Siyang | Leaves of greenhouse vegetables | 0.457b FW | 0.873b FW | 2.42b FW | NA | 1.68b FW | NA | 6.12b FW | - | [61] |
| Shenyang | 0.225b FW | 0.235b FW | 1.15b FW | NA | 0.81b FW | NA | 3.32b FW | - | [61] | |
| Beijing | 0.142b FW | 0.118b FW | 1.16b FW | NA | 1.25b FW | NA | 3.53b FW | - | [61] | |
| Shouguang | 0.159b FW | 0.106b FW | 1.01b FW | NA | 1.31b FW | NA | 2.95b FW | - | [61] | |
| Xianyang | 0.148b FW | 0.140b FW | 0.50b FW | NA | 1.04b FW | NA | 2.48b FW | - | [61] | |
| Haimen | 0.486b FW | 0.282b FW | 1.26b FW | NA | 0.82b FW | NA | 3.38b FW | - | [61] | |
| Nanjing | 0.394b FW | 0.446b FW | 1.92b FW | NA | 1.03b FW | NA | 4.81b FW | - | [61] | |
| Changshu | 0.182b FW | 0.219b FW | 2.10b FW | NA | 1.41b FW | NA | 4.91b FW | - | [61] | |
| Fuzhou | 0.193b FW | 0.188b FW | 0.76b FW | NA | 1.14b FW | NA | 3.36b FW | - | [61] | |
| Kunming | 0.156b FW | 0.152b FW | 0.80b FW | NA | 1.24b FW | NA | 2.90b FW | - | [61] | |
| Yellow River Delta | Vegetables | ND–0.036 | ND–0.063 | ND–1.300 | ND–0.034 | 0.002–15.700 | ND–0.154 | 0.011–16.400 | - | [25] |
| The coast of South China | Plant | 0.004–1.196 | 0.098–1.003 | 0.028–16.273 | ND–0.277 | 0.696–11.080 | ND–0.046 | 1.329–28.684 | 2.175–30.275 | This study |
3.4. Ecological and Human Risk Assessments of PAEs in Coastal Areas of South China
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bao, L.-J.; Wei, Y.-L.; Yao, Y.; Ruan, Q.-Q.; Zeng, E.Y. Global trends of research on emerging contaminants in the environment and humans: A literature assimilation. Environ. Sci. Pollut. Res. Int. 2015, 22, 1635–1643. [Google Scholar] [CrossRef]
- Tian, M.; Liu, L.; Wang, H.; Wang, X.; Martin, F.L.; Zhang, J.; Huang, Q.; Shen, H. Phthalates Induce Androgenic Effects at Exposure Levels That Can Be Environmentally Relevant in Humans. Environ. Sci. Technol. Lett. 2018, 5, 232–236. [Google Scholar] [CrossRef]
- Wang, W.X.; Zhang, Y.L.; Wang, S.L.; Fan, C.B.Q.; Xu, H. Distributions of phthalic esters carried by total suspended particulates in Nanjing, China. Environ. Monit. Assess. 2012, 184, 6789–6798. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.W.; Shi, J.H.; Bo, T.; Zhang, H.; Wu, W.; Chen, Q.C.; Zhan, X.M. Occurrence of phthalic acid esters in source waters: A nationwide survey in China during the period of 2009–2012. Environ. Pollut. 2014, 184, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liang, Q.; Gao, R.T.; Hou, H.B.; Tan, W.B.; He, X.S.; Zhang, H.; Yu, M.D.; Ma, L.N.; Xi, B.D.; et al. Contamination of Phthalate Esters (PAEs) in Typical Wastewater-Irrigated Agricultural Soils in Hebei, North China. PLoS ONE 2015, 10, e0137998. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liang, H.; Gao, D.W. Occurrence and risk assessment of phthalate esters (PAEs) in agricultural soils of the Sanjiang Plain, northeast China. Environ. Sci. Pollut. Res. Int. 2017, 24, 19723–19732. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, G.H.; Gu, H.; Huang, Q.Z.; Lou, C.H.; Zhang, L.; Liu, H.L. Assessing the Risk of Phthalate Ester (PAE) Contamination in Soils and Crops Irrigated with Treated Sewage Effluent. Water 2018, 10, 999. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.M.; Zhang, H.H.; Zou, Y.W.; Yang, G.P. Distribution and ecotoxicological state of phthalate esters in the sea-surface microlayer, seawater and sediment of the Bohai Sea and the Yellow Sea. Environ. Pollut. 2018, 240, 235–247. [Google Scholar] [CrossRef]
- Zhu, Q.Q.; Xu, L.Y.; Wang, W.Y.; Liu, W.B.; Liao, C.Y.; Jiang, G.B. Occurrence, spatial distribution and ecological risk assessment of phthalate esters in water, soil and sediment from Yangtze River Delta, China. Sci. Total Environ. 2022, 806, 1–8. [Google Scholar] [CrossRef]
- Ma, T.T.; Christie, P.; Luo, Y.M.; Teng, Y. Phthalate esters contamination in soil and plants on agricultural land near an electronic waste recycling site. Environ. Geochem. Health 2013, 35, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yan, H.Q.; Liu, Q.Y.; Li, X.Q.; Ge, J.; Yu, X.Y. Accumulation and transport patterns of six phthalic acid esters (PAEs) in two leafy vegetables under hydroponic conditions. Chemosphere 2020, 249, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sakhi, A.K.; Lillegaard, I.T.L.; Voorspoels, S.; Carlsen, M.H.; Loken, E.B.; Brantsaeter, A.L.; Haugen, M.; Meltzer, H.M.; Thomsen, C. Concentrations of phthalates and bisphenol A in Norwegian foods and beverages and estimated dietary exposure in adults. Environ. Int. 2014, 73, 259–269. [Google Scholar] [CrossRef]
- Zhang, A.; Liu, W.; Yuan, H.; Zhou, S.; Su, Y.; Li, Y.-F. Spatial Distribution of Hexachlorocyclohexanes in Agricultural Soils in Zhejiang Province, China, and Correlations with Elevation and Temperature. Environ. Sci. Technol. 2011, 45, 6303–6308. [Google Scholar] [CrossRef]
- Cai, Q.-Y.; Mo, C.-H.; Wu, Q.-T.; Katsoyiannis, A.; Zeng, Q.-Y. The status of soil contamination by semivolatile organic chemicals (SVOCs) in China: A review. Sci. Total Environ. 2008, 389, 209–224. [Google Scholar] [CrossRef] [PubMed]
- Net, S.; Sempere, R.; Delmont, A.; Paluselli, A.; Ouddane, B. Occurrence, Fate, Behavior and Ecotoxicological State of Phthalates in Different Environmental Matrices. Environ. Sci. Technol. 2015, 49, 4019–4035. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.Y.; Wen, B.; Shan, X.Q. Survey of phthalate pollution in arable soils in China. J. Environ. Monit. 2003, 5, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.T.; Pan, L.L.; Zhan, Y.; Lu, H.N.; Tsang, D.C.W.; Liu, W.X.; Wang, X.L.; Li, X.D.; Zhu, L.Z. Contamination of phthalate esters, organochlorine pesticides and polybrominated diphenyl ethers in agricultural soils from the Yangtze River Delta of China. Sci. Total Environ. 2016, 544, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, P.; Wang, L.; Sun, G.; Zhao, J.; Zhang, H.; Du, N. The influence of facility agriculture production on phthalate esters distribution in black soils of northeast China. Sci. Total Environ. 2015, 506–507, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, G.; Christie, P.; Zhang, M.; Luo, Y.; Teng, Y. Occurrence and risk assessment of phthalate esters (PAEs) in vegetables and soils of suburban plastic film greenhouses. Sci. Total Environ. 2015, 523, 129–137. [Google Scholar] [CrossRef]
- Tsai, H.-J.; Chen, B.-H.; Wu, C.-F.; Wang, S.-L.; Huang, P.-C.; Tsai, Y.-C.; Chen, M.-L.; Ho, C.-K.; Hsiung, C.A.; Wu, M.-T. Intake of phthalate-tainted foods and microalbuminuria in children: The 2011 Taiwan food scandal. Environ. Int. 2016, 89–90, 129–137. [Google Scholar] [CrossRef]
- Poma, G.; Glynn, A.; Malarvannan, G.; Covaci, A.; Darnerud, P.O. Dietary intake of phosphorus flame retardants (PFRs) using Swedish food market basket estimations. Food Chem. Toxicol. 2017, 100, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Gao, M.L.; Dong, Y.M.; Zhang, Z.; Song, Z.G. Metabolism and distribution of dibutyl phthalate in wheat grown on different soil types. Chemosphere 2019, 236, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.J.; Wang, Y.T.; Huang, Y.W.; Liu, F.; Teng, Y. Uptake, translocation and metabolism of di-n-butyl phthalate in alfalfa (Medicago sativa). Sci. Total Environ. 2020, 731, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-W.; Cai, Q.-Y.; Mo, C.-H.; Zeng, Q.-Y.; Lu, H.; Li, Q.-S.; Xu, G.-S. Plant Uptake and Enhanced Dissipation of Di(2-Ethylhexyl) Phthalate (DEHP) in Spiked Soils by Different Plant Species. Int. J. Phytorem. 2014, 16, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Li, Z.; Sun, J.; Zhu, L. Pollution characteristics and health risk assessment of phthalate esters in agricultural soil and vegetables in the Yangtze River Delta of China. Sci. Total Environ. 2020, 726, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xi, Y.; Shi, X.-Y.; Zhong, Y.-J.; Guo, C.-L.; Han, Y.-N.; Li, F.-M. Effect of plastic film mulching and film residues on phthalate esters concentrations in soil and plants, and its risk assessment. Environ. Pollut. 2021, 286, 1–8. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Bao, M.; Xu, Y.; Zhang, L.; Tan, F.; Zhao, H. Characteristics and risk assessment of organophosphate esters and phthalates in soils and vegetation from Dalian, northeast China. Environ. Pollut. 2021, 284, 1–7. [Google Scholar] [CrossRef]
- Kimber, I.; Dearman, R.J. An assessment of the ability of phthalates to influence immune and allergic responses. Toxicology 2010, 271, 73–82. [Google Scholar] [CrossRef]
- Jugan, M.L.; Levi, Y.; Blondeau, J.P. Endocrine disruptors and thyroid hormone physiology. Biochem. Pharmacol. 2010, 79, 939–947. [Google Scholar] [CrossRef] [Green Version]
- Maqbool, F.; Mostafalou, S.; Bahadar, H.; Abdollahi, M. Review of endocrine disorders associated with environmental toxicants and possible involved mechanisms. Life Sci. 2016, 145, 265–273. [Google Scholar] [CrossRef]
- Casals-Casas, C.; Desvergne, B. Endocrine Disruptors: From Endocrine to Metabolic Disruption. Annu. Rev. Physiol. 2011, 73, 135–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benjamin, S.; Masai, E.; Kamimura, N.; Takahashi, K.; Anderson, R.C.; Faisal, P.A. Phthalates impact human health: Epidemiological evidences and plausible mechanism of action. J. Hazard. Mater. 2017, 340, 360–383. [Google Scholar] [CrossRef] [PubMed]
- Dou, P.-C.; Mai, L.; Bao, L.-J.; Zeng, E.Y. Microplastics on beaches and mangrove sediments along the coast of South China. Mar. Pollut. Bull. 2021, 172, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Wang, P.; Wu, S.; Luo, M. Urbanization effects on high-frequency temperature variability over South China. Urban. Clim. 2022, 42, 1–13. [Google Scholar] [CrossRef]
- Ma, L.L.; Chu, S.G.; Xu, X.B. Organic contamination in the greenhouse soils from Beijing suburbs, China. J. Environ. Monit. 2003, 5, 786–790. [Google Scholar] [CrossRef] [PubMed]
- USEPA. Electronic Code of Federal Regulations, Title 40-Protection of Environment, Part 423d Steam Electric Power Generating Point Source Category. Appendix A to Part 423-126, Priority Pollutants; USEPA: Washington, DC, USA, 2013.
- Zhou, B.; Zhao, L.; Sun, Y.; Li, X.; Weng, L.; Li, Y. Contamination and human health risks of phthalate esters in vegetable and crop soils from the Huang-Huai-Hai region of China. Sci. Total Environ. 2021, 778, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Pan, L.; Tsang, D.C.W.; Zhan, Y.; Liu, W.; Wang, X.; Zhu, L.; Li, X. Polychlorinated biphenyls in agricultural soils from the Yangtze River Delta of China: Regional contamination characteristics, combined ecological effects and human health risks. Chemosphere 2016, 163, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.-H.; Zhang, H.-X.; Wu, X.; Gu, H.-X.; Zhang, L.-Z.; Liu, Y.-Y.; Zhao, X.-F.; Li, J.-H. Pollution levels and health risk assessment of particulate phthalic acid esters in arid urban areas. Atmos. Pollut. Res. 2017, 8, 188–195. [Google Scholar] [CrossRef]
- Luo, Q.; Liu, Z.-h.; Yin, H.; Dang, Z.; Wu, P.-x.; Zhu, N.-w.; Lin, Z.; Liu, Y. Migration and potential risk of trace phthalates in bottled water: A global situation. Water. Res. 2018, 147, 362–372. [Google Scholar] [CrossRef]
- Yang, H.J.; Xie, W.J.; Liu, Q.; Liu, J.T.; Yu, H.W.; Lu, Z.H. Distribution of phthalate esters in topsoil: A case study in the Yellow River Delta, China. Environ. Monit. Assess. 2013, 185, 8489–8500. [Google Scholar]
- Tao, H.; Wang, Y.; Liang, H.; Zhang, X.; Liu, X.; Li, J. Pollution characteristics of phthalate acid esters in agricultural soil of Yinchuan, northwest China, and health risk assessment. Environ. Geochem. Health 2020, 42, 4313–4326. [Google Scholar] [CrossRef]
- Li, K.; Ma, D.; Wu, J.; Chai, C.; Shi, Y. Distribution of phthalate esters in agricultural soil with plastic film mulching in Shandong Peninsula, East China. Chemosphere 2016, 164, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, X.; Lu, X. Phthalic acid esters (PAEs) in vegetable soil from the suburbs of Xianyang city, Northwest China. Environ. Earth. Sci. 2015, 74, 1487–1496. [Google Scholar] [CrossRef]
- Mo, C.-H.; Cai, Q.-Y.; Li, Y.-H.; Zeng, Q.-Y. Occurrence of priority organic pollutants in the fertilizers, China. J. Hazard. Mater. 2008, 152, 1208–1213. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Xu, Y.; Xu, C.; Yun, L.; Liu, W. Status of phthalate esters contamination in agricultural soils across China and associated health risks. Environ. Pollut. 2014, 195, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Li, F.H.; Wang, Q.H. Occurrence and degradation characteristics of dibutyl phthalate (DBP) and di-(2-ethylhexyl) phthalate (DEHP) in typical agricultural soils of China. Sci. Total Environ. 2008, 393, 333–340. [Google Scholar] [CrossRef]
- Kong, S.F.; Ji, Y.Q.; Liu, L.L.; Chen, L.; Zhao, X.Y.; Wang, J.J.; Bai, Z.P.; Sun, Z.R. Diversities of phthalate esters in suburban agricultural soils and wasteland soil appeared with urbanization in China. Environ. Pollut. 2012, 170, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Lin, Q.X.; Wang, J.; Lu, X.G.; Wang, G.P. Effect of wetland reclamation and tillage conversion on accumulation and distribution of phthalate esters residues in soils. Ecol. Eng. 2013, 51, 10–15. [Google Scholar] [CrossRef]
- Wang, J.; Luo, Y.M.; Teng, Y.; Ma, W.T.; Christie, P.; Li, Z.G. Soil contamination by phthalate esters in Chinese intensive vegetable production systems with different modes of use of plastic film. Environ. Pollut. 2013, 180, 265–273. [Google Scholar] [CrossRef]
- Peijnenburg, W.J.G.M.; Struijs, J. Occurrence of phthalate esters in the environment of the Netherlands. Ecotoxicol. Environ. Saf. 2006, 63, 204–215. [Google Scholar] [CrossRef] [Green Version]
- Rhind, S.M.; Kyle, C.E.; Kerr, C.; Osprey, M.; Zhang, Z.L.; Duff, E.I.; Lilly, A.; Nolan, A.; Hudson, G.; Towers, W.; et al. Concentrations and geographic distribution of selected organic pollutants in Scottish surface soils. Environ. Pollut. 2013, 182, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Škrbić, B.D.; Ji, Y.; Đurišić-Mladenović, N.; Zhao, J. Occurence of the phthalate esters in soil and street dust samples from the Novi Sad city area, Serbia, and the influence on the children’s and adults’ exposure. J. Hazard. Mater. 2016, 312, 272–279. [Google Scholar] [CrossRef]
- Mo, C.H.; Cai, Q.Y.; Tang, S.R.; Zeng, Q.Y.; Wu, Q.T. Polycyclic Aromatic Hydrocarbons and Phthalic Acid Esters in Vegetables from Nine Farms of the Pearl River Delta, South China. Arch. Environ. Contam. Toxicol. 2009, 56, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Muchangos, L.d.; Xue, M.; Zhou, L.; Kojima, N.; Machimura, T.; Tokai, A. Flows, stocks, and emissions of DEHP products in Japan. Sci. Total Environ. 2019, 650, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- Chao, W.L.; Cheng, C.Y. Effect of introduced phthalate-degrading bacteria on the diversity of indigenous bacterial communities during di-(2-ethylhexyl) phthalate (DEHP) degradation in a soil microcosm. Chemosphere 2007, 67, 482–488. [Google Scholar] [CrossRef]
- Liu, H.; Liang, H.; Liang, Y.; Zhang, D.; Wang, C.; Cai, H.; Shvartsev, S.L. Distribution of phthalate esters in alluvial sediment: A case study at JiangHan Plain, Central China. Chemosphere 2010, 78, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhang, X.; Wu, X.; Shen, G.; Du, Q.; Mo, C. Uptake of Di(2-ethylhexyl) Phthalate (DEHP) by the Plant Benincasa hispida and Its Use for Lowering DEHP Content of Intercropped Vegetables. J. Agric. Food Chem. 2013, 61, 5220–5225. [Google Scholar] [CrossRef]
- He, L.; Gielen, G.; Bolan, N.S.; Zhang, X.; Qin, H.; Huang, H.; Wang, H. Contamination and remediation of phthalic acid esters in agricultural soils in China: A review. Agron. Sustain. Dev. 2015, 35, 519–534. [Google Scholar] [CrossRef]
- Du, Q.; Shen, L.; Xiu, L.; Jerz, G.; Winterhalter, P. Di-2-ethylhexyl phthalate in the fruits of Benincasa hispida. Food Addit. Contam. A 2006, 23, 552–555. [Google Scholar] [CrossRef]
- Chen, N.; Shuai, W.; Hao, X.; Zhang, H.; Zhou, D.; Gao, J. Contamination of Phthalate Esters in Vegetable Agriculture and Human Cumulative Risk Assessment. Pedosphere 2017, 27, 439–451. [Google Scholar] [CrossRef]
- Zhu, T.-K.; Du, P.-P.; Zeng, L.-J.; Lü, H.; Zhao, H.-M.; Li, Y.-W.; Mo, C.-H.; Cai, Q.-Y. Variation in metabolism and degradation of di-n-butyl phthalate (DBP) by high- and low-DBP accumulating cultivars of rice (Oryza sativa L.) and crude enzyme extracts. Sci. Total Environ. 2019, 668, 1117–1127. [Google Scholar] [CrossRef]
- Zhang, S.-h.; Guo, A.-j.; Fan, T.-t.; Zhang, R.; Niu, Y.-j. Phthalates in residential and agricultural soils from an electronic waste-polluted region in South China: Distribution, compositional profile and sources. Environ. Sci. Pollut. Res. 2019, 26, 12227–12236. [Google Scholar] [CrossRef]
- Nas, B.; Ates, H.; Dolu, T.; Yel, E.; Argun, M.E.; Koyuncu, S.; Kara, M.; Dinc, S. Evaluation of occurrence, fate and removal of priority phthalate esters (PAEs) in wastewater and sewage sludge by advanced biological treatment, waste stabilization pond and constructed wetland. Chemosphere 2022, 295, 1–12. [Google Scholar] [CrossRef]
- Gao, D.; Li, Z.; Wang, H.; Liang, H. An overview of phthalate acid ester pollution in China over the last decade: Environmental occurrence and human exposure. Sci. Total Environ. 2018, 645, 1400–1409. [Google Scholar] [CrossRef]
- Liu, S.; Peng, Y.; Lin, Q.; Xiao, R.; Luo, H.; Liao, X.; Yin, G.; Liu, Q. Di-(2-Ethylhexyl) Phthalate as a Chemical Indicator for Phthalic Acid Esters: An Investigation into Phthalic Acid Esters in Cultivated Fields and E-Waste Dismantling Sites. Environ. Toxicol. Chem. 2019, 38, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- USEPA. Mid-Atlantic RISK Assessment; Untied States Environmental Protection Agency: Washington, DC, USA, 2015.
- USEPA. Exposure Factors Handbook, EPA/600/P-95/002F; Environmental Protection Agency; Office of Research and Development: Washington, DC, USA, 1997.
- China N.B.o.S.o. China Statistical Yearbook; China Statistic Press: Beijing, China, 2015.
- Zeng, L.-J.; Huang, Y.-H.; Chen, X.-T.; Chen, X.-H.; Mo, C.-H.; Feng, Y.-X.; Lü, H.; Xiang, L.; Li, Y.-W.; Li, H.; et al. Prevalent phthalates in air-soil-vegetable systems of plastic greenhouses in a subtropical city and health risk assessments. Sci. Total Environ. 2020, 743, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, M.; Tao, W.; Zhang, W.; Wang, L.; Shi, X.; Lu, X.; Li, X. Pollution characteristics and health risk assessment of phthalate esters in urban soil in the typical semi-arid city of Xi’an, Northwest China. Chemosphere 2018, 191, 467–476. [Google Scholar] [CrossRef]




| Compound | Detection Rate (%) | Mean (mg/kg) | Median (mg/kg) | Min (mg/kg) | Max (mg/kg) |
|---|---|---|---|---|---|
| DEHP | 100 | 0.947 | 0.783 | 0.129 | 2.628 |
| DnBP | 100 | 0.259 | 0.228 | 0.073 | 1.109 |
| DiBP | 100 | 0.121 | 0.095 | 0.030 | 0.861 |
| DEP | 100 | 0.060 | 0.048 | 0.004 | 0.364 |
| DMP | 100 | 0.054 | 0.050 | 0.003 | 0.319 |
| BMPP | 89 | 0.006 | 0.002 | ND a | 0.087 |
| DMEP | 86 | 0.007 | 0.0004 | ND | 0.042 |
| DEEP | 86 | 0.005 | 0.002 | ND | 0.016 |
| DnHP | 78 | 0.053 | 0.001 | ND | 1.449 |
| DnOP | 76 | 0.006 | 0.003 | ND | 0.054 |
| DBEP | 73 | 0.052 | 0.008 | ND | 0.753 |
| DNP | 70 | 0.003 | 0.002 | ND | 0.021 |
| DPP | 70 | 0.003 | 0.001 | ND | 0.010 |
| BBP | 59 | 0.004 | 0.001 | ND | 0.024 |
| DCHP | 41 | 0.005 | ND | ND | 0.072 |
| Σ6 PAEs | 100 | 1.329 | 1.182 | 0.239 | 3.495 |
| Σ15 PAEs | 100 | 1.582 | 1.272 | 0.445 | 4.437 |
| Location | Soil Types | DMP | DEP | DnBP | BBP | DEHP | DnOP | Σ6 PAEs | Σ15 PAEs | References |
|---|---|---|---|---|---|---|---|---|---|---|
| East China (7 cites) | Arable soils | ND | ND–1.29 | 0.21–1.38 | NA | 0.2–5.98 | NA | 1.34–7.14 | - | [16] |
| Northeast China (3 cities) | Arable soils | ND | 0.18–1.36 | 0.16–1.56 | NA | 0.51–2.15 | NA | 4.41–10.03 | - | [16] |
| North China (4 cities) | Arable soils | ND–0.2 | 0.15–2.61 | 0.14–0.98 | NA | 0.51–2.18 | NA | 1.76–3.78 | - | [16] |
| Northwest China (2 cities) | Arable soils | ND | 0.18–0.25 | 0.38–0.39 | NA | 1.67–2.17 | NA | 2.23–2.81 | - | [16] |
| South China (4 cities) | Arable soils | ND | ND–0.17 | ND–0.26 | NA | 0.54–3.42 | NA | 0.89–3.16 | - | [16] |
| Southwest China (3 cities) | Arable soils | ND | ND–0.37 | 0.51–0.64 | NA | 1.02–2.08 | NA | 1.85–2.96 | - | [16] |
| Harbin | Black soils | NA | NA | 2.75–14.62 | NA | 0.49–4.2 | NA | - | - | [47] |
| Handan | Fluvo-aquic soils | NA | NA | 3.18–29.37 | NA | 1.15–7.99 | NA | - | - | [47] |
| Beijing | Greenhouse soils | 0.01–0.02 | 0.01–0.05 | 0.34–1.66 | NA | 0.22–0.74 | ND–0.09 | 1.34–3.15 | - | [35] |
| Tianjin | Farmland soils | 0.003–0.088 | 0.003–0.081 | 0.007–0.189 | ND–1.79 | 0.039–2.37 | ND–0.647 | 0.091–2.74 | - | [48] |
| Vegetable soils | 0.002–0.101 | 0.002–0.114 | 0.013–0.285 | ND–0.358 | 0.028–4.17 | ND–9.78 | 0.050–10.4 | - | [48] | |
| Orchard soils | 0.003–0.032 | 0.003–0.03 | 0.02–0.138 | ND–0.125 | 0.026–0.358 | ND–0.728 | 0.053–1.08 | - | [48] | |
| Wasteland soils | 0.003–0.073 | 0.005–0.059 | 0.009–0.147 | ND–0.471 | 0.051–0.494 | ND–1.00 | 0.106–1.36 | - | [48] | |
| Sanjiang Plain | Cultivated topsoils | 0.0266b | 0.0349b | 0.0285b | NA | 0.0279b | NA | - | - | [49] |
| Yellow River Delta | Urban soils | 0.002–0.060 | ND–0.004 | 0.245–2.058 | NA | 1.465–6.320 | ND–0.044 | 1.987–8.454 | Σ11 PAEs: 2.096–8.527 | [41] |
| Suburb soils | 0.001–0.065 | 0.001–11.24 | 0.166–1.450 | NA | 0.710–4.473 | ND–0.142 | 1.007–16.007 | 1.079–19.504 | [41] | |
| Rural soils | 0.001–0.005 | ND–0.001 | 0.136–1.039 | NA | 0.431–2.449 | ND–0.068 | 0.716–3.251 | 0.794–3.461 | [41] | |
| Nanjing | Vegetable soils | ND–0.012 | ND–0.007 | ND–0.046 | ND–0.002 | 0.204–0.704 | 0.002–0.019 | 0.314–0.564 | - | [50] |
| Using plastic soils | ND–0.016 | ND–0.027 | ND–1.41 | ND–0.041 | 0.034–7.033 | ND–1.739 | 0.480–9.676 | - | [50] | |
| Yellow River Delta | Agriculture soils | 0.002–0.071 | 0.0005–0.0906 | ND–1.5 | ND–0.0122 | ND–9.91 | ND–0.274 | 0.068–9.33 | Σ15PAEs: 0.167–9.37 | [17] |
| Yellow River Delta | Agriculture soils | ND–0.002 | ND–0.004 | ND–0.069 | ND–0.096 | 0.004–1.510 | ND–0.075 | 0.005–1.580 | - | [25] |
| Shandong Peninsula | Agriculture soils | ND–1.179 | 0.010–1.900 | ND–9.855 | ND–4.786 | ND–2.943 | ND–5.873 | - | Σ16 PAEs: 1.374–18.810 | [43] |
| Yinchuan | Agriculture soils | 0.089–4.684 | 0.004–6.017 | 0.018–2.653 | ND–0.039 | 0.069–2.693 | ND–1.354 | 0.374–11.659 | Σ16 PAEs: 0.391–11.924 | [42] |
| Netherland | Soils | NA | NA | 0.006b | NA | 0.0318b | NA | 0.038b | - | [51] |
| Scotland | Surface soils | NA | NA | NA | NA | 0.025–1.60 | NA | - | - | [52] |
| Serbia | Surface soils | 0.006–0.038 | 0.005–0.011 | 0.030–0.145 | 0.001–0.013 | 0.13–2.04 | 0.0004–0.013 | 0.19–2.12 | - | [53] |
| Xianyang | Vegetable soils | 0.0213–0.0823 | ND–0.067 | 0.037–6.313 | ND–0.222 | ND–3.871 | ND–0.763 | 0.129–10.288 | - | [44] |
| Huang-Huai-Hai | Agriculture soils | ND–0.116 | ND–0.622 | ND–1.417 | ND–0.688 | ND–2.314 | ND–0.606 | 0.046–3.423 | Σ16 PAEs: 0.0517–3.569 | [37] |
| The coast of South China | Agriculture soils | 0.003–0.319 | 0.004–0.364 | 0.073–1.109 | ND–0.024 | 0.129–2.628 | ND–0.054 | 0.239–3.495 | 0.445–4.437 | This study |
| Compound | Detection Rate (%) | Mean (mg/kg) | Median (mg/kg) | Min (mg/kg) | Max (mg/kg) |
|---|---|---|---|---|---|
| DEHP | 100 | 4.114 | 2.896 | 0.696 | 11.081 |
| DnBP | 100 | 2.282 | 1.544 | 0.028 | 16.273 |
| DiBP | 100 | 1.139 | 0.778 | 0.110 | 7.916 |
| DEP | 100 | 0.292 | 0.214 | 0.098 | 1.003 |
| DMP | 100 | 0.374 | 0.340 | 0.004 | 1.196 |
| BMPP | 86 | 0.130 | 0.080 | ND a | 0.737 |
| DMEP | 78 | 0.121 | 0.144 | ND | 0.286 |
| DEEP | 51 | 0.082 | 0.011 | ND | 0.690 |
| DnHP | 43 | 0.114 | ND | ND | 1.917 |
| DnOP | 35 | 0.005 | ND | ND | 0.046 |
| DBEP | 22 | 0.006 | ND | ND | 0.046 |
| DNP | 16 | 0.019 | ND | ND | 0.277 |
| DPP | 16 | 0.014 | ND | ND | 0.201 |
| BBP | 5 | 0.004 | ND | ND | 0.090 |
| DCHP | 3 | 0.004 | ND | ND | 0.158 |
| Σ6 PAEs | 100 | 7.087 | 4.985 | 1.329 | 28.685 |
| Σ15 PAEs | 100 | 8.712 | 6.505 | 2.176 | 30.276 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, H.; Yu, X.; Huang, J.; Du, X.; Wang, M.; Sun, J.; Lu, G.; Tao, X. Characteristics and Health Risks of Phthalate Ester Contamination in Soil and Plants in Coastal Areas of South China. Int. J. Environ. Res. Public Health 2022, 19, 9516. https://doi.org/10.3390/ijerph19159516
Xing H, Yu X, Huang J, Du X, Wang M, Sun J, Lu G, Tao X. Characteristics and Health Risks of Phthalate Ester Contamination in Soil and Plants in Coastal Areas of South China. International Journal of Environmental Research and Public Health. 2022; 19(15):9516. https://doi.org/10.3390/ijerph19159516
Chicago/Turabian StyleXing, Huanhuan, Xiaolong Yu, Jiahui Huang, Xiaodong Du, Mengting Wang, Jianteng Sun, Guining Lu, and Xueqin Tao. 2022. "Characteristics and Health Risks of Phthalate Ester Contamination in Soil and Plants in Coastal Areas of South China" International Journal of Environmental Research and Public Health 19, no. 15: 9516. https://doi.org/10.3390/ijerph19159516
APA StyleXing, H., Yu, X., Huang, J., Du, X., Wang, M., Sun, J., Lu, G., & Tao, X. (2022). Characteristics and Health Risks of Phthalate Ester Contamination in Soil and Plants in Coastal Areas of South China. International Journal of Environmental Research and Public Health, 19(15), 9516. https://doi.org/10.3390/ijerph19159516