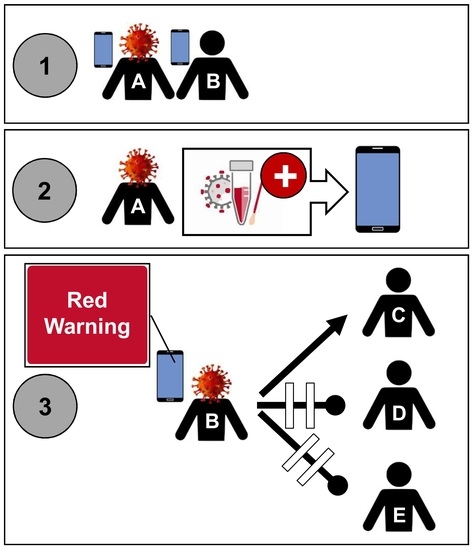
The German COVID-19 Digital Contact Tracing App: A Socioeconomic Evaluation
Abstract
:1. Introduction
- First, descriptive statistics provide information on how the CWA and its use have developed among the German population.
- Then, a utility analysis examines the effectiveness of the CWA. According to the federal government, the stated goal of the CWA is to quickly detect and interrupt chains of infection [14]. Whether it has met this goal is analyzed by modeling prevented infections, hospitalizations, intensive care treatments, and deaths.
- In the third step, the efficiency of the CWA is examined by means of a cost–benefit analysis. A societal economic welfare criterion is determined from monetized and summed effects and quantified via net present value (NPV) and benefit–cost ratio (BCR).
- The fourth step focuses on identifying and quantifying the factors with a crucial impact on the effectiveness and efficiency of the CWA.
2. Materials and Methods
2.1. Software and Statistics
2.2. Actuarial Assumptions
2.3. Calculation of the Effectiveness of the CWA
2.4. Reduction of the R-Value and the Number of Cases by the Corona Warn App
2.5. Cost Calculation
2.5.1. Development and Operation of the CWA
2.5.2. Costs of Testing
2.5.3. Costs Due to Continued Pension Payments
2.6. Benefit Calculation
2.6.1. Benefits from Reduced Loss of Earnings
2.6.2. Benefits from Reduced Hospitalizations and Intensive Care Treatments
2.6.3. Benefits from Reduced Rehabilitation Measures
2.6.4. Benefits from Reduced Deaths
2.7. Net Present Value and Benefit–Cost Ratio
2.8. Sensitivity Analysis
3. Results
3.1. Descriptive Statistics
3.1.1. Development of Case Numbers during the Observation Period
3.1.2. Development of the Adoption Rate
3.1.3. Reduction in the R-Value
3.2. Utility Analysis
3.3. Cost–Benefit Analysis
3.4. Results of the One-at-a-Time Sensitivity Analysis
4. Discussion
4.1. Related Work
4.2. Limitations
4.3. Intangible Effects
- The reduction in the number of cases of infection caused by the CWA also led to a reduction in suffering—on the one hand, suffering for the patients themselves and, on the other hand, also for their relatives, especially in the case of an otherwise fatal course.
- At least for long COVID cases, but also for other severe courses, the prevention of infection is likely to have contributed to increased quality of life in the longer term.
- The CWA has helped to introduce electronic health solutions on a broad scale and has paved the way for digital health solutions to become part of everyday life.
- The CWA offers a scalable approach, unlike manual contact tracking. German health authorities reach the limits of manual contact tracing at incidences between 35 and 50/100,000 [86]. This fact could only be met to a limited extent and, above all, only approximately linearly by hiring more staff—even by a logistically hardly possible doubling of the staff, only infection cases up to an approximate incidence of 100/100,000 could be followed up manually. DCT apps have no limits even at maximum incidences.
- The concept of the CWA can be used for other pandemics or epidemics in the future with manageable adaptations. There is no doubt that there will be more severe courses of seasonal influenza in the coming years, and another pandemic will almost certainly occur again [87].
4.4. Reasons for an Overly Optimistic Assessment
4.5. Reasons for an Overly Pessimistic Assessment
4.6. Possible Optimizations of the Corona Warn App
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, X.; Lau, E.H.Y.; Wu, P.; Deng, X.; Wang, J.; Hao, X.; Lau, Y.C.; Wong, J.Y.; Guan, Y.; Tan, X.; et al. Temporal Dynamics in Viral Shedding and Transmissibility of COVID-19. Nat. Med. 2020, 26, 672–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferretti, L.; Wymant, C.; Kendall, M.; Zhao, L.; Nurtay, A.; Abeler-Dörner, L.; Parker, M.; Bonsall, D.; Fraser, C. Quantifying SARS-CoV-2 Transmission Suggests Epidemic Control with Digital Contact Tracing. Science 2020, 368, 6491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitelaw, S.; Mamas, M.A.; Topol, E.; van Spall, H.G.C. Applications of Digital Technology in COVID-19 Pandemic Planning and Response. Lancet Digit Health 2020, 2, e435–e440. [Google Scholar] [CrossRef]
- el Orden Mundial. Las Apps de Rastreo En Europa, Un Fracaso de Más de Cien Millones de Euros. 27 December 2021. Available online: https://elordenmundial.com/las-apps-de-rastreo-en-europa-un-fracaso-de-mas-de-cien-millones-de-euros/ (accessed on 10 April 2022).
- Bundesregierung. Antwort Der Bundesregierung Auf Die Kleine Anfrage Der Abgeordneten René Springer, Gerrit Huy, Jörg Schneider, Weiterer Abgeordneter Und Der Fraktion Der AfD—Drucksache 20/224. 2022. Available online: https://dserver.bundestag.de/btd/20/004/2000431.pdf (accessed on 29 March 2022).
- Munzert, S.; Selb, P.; Gohdes, A.; Stoetzer, L.F.; Lowe, W. Tracking and Promoting the Usage of a COVID-19 Contact Tracing App. Nat. Hum. Behav. 2021, 5, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Alshawi, A.; Al-Razgan, M.; AlKallas, F.H.; Suhaim, R.A.b.; Al-Tamimi, R.; Alharbi, N.; AlSaif, S.O. Data Privacy during Pandemics: A Systematic Literature Review of COVID-19 Smartphone Applications. PeerJ Comput. Sci. 2022, 8, e826. [Google Scholar] [CrossRef]
- Grill, E.; Eitze, S.; de Bock, F.; Dragano, N.; Huebl, L.; Schmich, P.; Wieler, L.H.; Betsch, C. Sociodemographic Characteristics Determine Download and Use of a Corona Contact Tracing App in Germany-Results of the COSMO Surveys. PLoS ONE 2021, 16, e0256660. [Google Scholar] [CrossRef]
- Abramova, O.; Wagner, A.; Olt, C.M.; Buxmann, P. One for All, All for One: Social Considerations in User Acceptance of Contact Tracing Apps Using Longitudinal Evidence from Germany and Switzerland. Int. J. Inf. Manage 2022, 64, 102473. [Google Scholar] [CrossRef]
- Kretzschmar, M.E.; Rozhnova, G.; Bootsma, M.C.J.; van Boven, M.; van de Wijgert, J.H.H.M.; Bonten, M.J.M. Impact of Delays on Effectiveness of Contact Tracing Strategies for COVID-19: A Modelling Study. Lancet Public Health 2020, 5, e452–e459. [Google Scholar] [CrossRef]
- Kucharski, A.J.; Klepac, P.; Conlan, A.J.K.; Kissler, S.M.; Tang, M.L.; Fry, H.; Gog, J.R.; Edmunds, W.J.; Emery, J.C.; Medley, G.; et al. Effectiveness of Isolation, Testing, Contact Tracing, and Physical Distancing on Reducing Transmission of SARS-CoV-2 in Different Settings: A Mathematical Modelling Study. Lancet Infect Dis. 2020, 20, 1151–1160. [Google Scholar] [CrossRef]
- Plank, M.J.; James, A.; Lustig, A.; Steyn, N.; Binny, R.N.; Hendy, S.C. Potential Reduction in Transmission of COVID-19 by Digital Contact Tracing Systems: A Modelling Study. medRxiv 2021. [Google Scholar] [CrossRef]
- Elmokashfi, A.; Sundnes, J.; Kvalbein, A.; Naumova, V.; Reinemo, S.-A.A.; Magne Florvaag, P.; Kvale Stensland, H.; Lysne, O.; Florvaag, P.M.; Stensland, H.K.; et al. Nationwide Rollout Reveals Efficacy of Epidemic Control through Digital Contact Tracing. Nat. Commun. 2021, 12, 5918. [Google Scholar] [CrossRef] [PubMed]
- Bundesregierung. Corona-Warn-App: Fragen Und Antworten. 29 November 2021. Available online: https://www.bundesregierung.de/breg-de/themen/corona-warn-app/corona-warn-app-faq-1758392 (accessed on 15 April 2022).
- Bundesministerium der Finanzen. Personalkosten, Sachkosten Und Kalkulationszinssätze in Der Bundesverwaltung Für Kostenberechnungen Und Wirtschaftlichkeitsuntersuchungen. 2020. Available online: https://www.bundesfinanzministerium.de/Content/DE/Standardartikel/Themen/Oeffentliche_Finanzen/Bundeshaushalt/personalkostensaetze-2020-anl.pdf?__blob=publicationFile&v=2 (accessed on 15 April 2022).
- Dehmel, S.; Kenning, P.; Wagner, G.G.; Liedtke, C.; Micklitz, H.W.; Specht-Riemenschneider, L. Die Wirksamkeit Der Corona-Warn-App Wird Sich Nur Im Praxistest Zeigen. Der Datenschutz Ist Nur Eine von Vielen Herausforderungen. Veröffentlichungen Sachverständigenrats Verbrauch. 2020, 1–37. [Google Scholar]
- Jenniskens, K.; Bootsma, M.C.J.; Damen, J.A.A.G.; Oerbekke, M.S.; Vernooij, R.W.M.; Spijker, R.; Moons, K.G.M.; Kretzschmar, M.E.E.; Hooft, L. Effectiveness of Contact Tracing Apps for SARS-CoV-2: A Rapid Systematic Review. BMJ Open 2021, 11, e050519. [Google Scholar] [CrossRef] [PubMed]
- Corona-Warn-App. Kennzahlen-Dashboard. Available online: https://www.coronawarn.app/de/analysis/ (accessed on 9 April 2022).
- Corona-Warn-App. Wie Viele Aktive Nutzende Hat Die Corona-Warn-App? Available online: https://www.coronawarn.app/de/science/2022-03-03-science-blog-5/ (accessed on 9 April 2022).
- Statista. Corona-Warn-App: Anzahl Potenziell Teilbarer Positiver Testergebnisse in Deutschland. 2021. Available online: https://de.statista.com/statistik/daten/studie/1179649/umfrage/anzahl-potenziell-teilbarer-positiver-testergebnisse/ (accessed on 13 April 2022).
- Flaxman, S.; Mishra, S.; Gandy, A.; Unwin, H.J.T.; Mellan, T.A.; Coupland, H.; Whittaker, C.; Zhu, H.; Berah, T.; Eaton, J.W.; et al. Estimating the Effects of Non-Pharmaceutical Interventions on COVID-19 in Europe. Nature 2020, 584, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.D.; Tekwa, E.W.; Gignoux-Wolfsohn, S.A. Social, Economic, and Environmental Factors Influencing the Basic Reproduction Number of COVID-19 across Countries. PLoS ONE 2021, 16, e0252373. [Google Scholar] [CrossRef]
- Li, Y.; Campbell, H.; Kulkarni, D.; Harpur, A.; Nundy, M.; Wang, X.; Nair, H. The Temporal Association of Introducing and Lifting Non-Pharmaceutical Interventions with the Time-Varying Reproduction Number (R) of SARS-CoV-2: A Modelling Study across 131 Countries. Lancet Infect Dis. 2021, 21, 193–202. [Google Scholar] [CrossRef]
- Corona-Warn-App. About the Effectiveness and Benefits of the Corona-Warn-App. Available online: https://www.coronawarn.app/en/science/2021-06-15-science-blog-1/#what-are-the-benefits-of-the-corona-warn-app (accessed on 9 April 2022).
- Our World in Data. Coronavirus (COVID-19) Testing. Available online: https://ourworldindata.org/coronavirus-testing (accessed on 7 April 2022).
- Kassenärztliche Bundesvereinigung. Anschluss an Die Corona-Warn-App Für Bürgertests Erforderlich. 7 August 2021. Available online: https://www.kbv.de/html/1150_53277.php (accessed on 11 April 2022).
- Kassenärztliche Bundesvereinigung. Start Der Corona-Warn-App: Neue EBM-Leistungen Zur Testung. 16 June 2020. Available online: https://www.kbv.de/html/1150_46657.php (accessed on 11 April 2022).
- Our World in Data. Coronavirus (COVID-19) Deaths. Available online: https://ourworldindata.org/covid-deaths#country-by-country-data-on-confirmed-deaths (accessed on 7 April 2022).
- an der Heiden, M. SARS-CoV-2-Nowcasting Und -R-Schätzung. 2022. [Google Scholar] [CrossRef]
- Statista. Coronavirus—Todesfälle in Deutschland Nach Alter. 30 March 2022. Available online: https://de.statista.com/statistik/daten/studie/1104173/umfrage/todesfaelle-aufgrund-des-coronavirus-in-deutschland-nach-geschlecht/ (accessed on 7 April 2022).
- Deutsche Rentenversicherung. Rentenversicherung in Zahlen. 2021, pp. 34–37. Available online: https://www.deutsche-rentenversicherung.de/SharedDocs/Downloads/DE/Statistiken-und-Berichte/statistikpublikationen/rv_in_zahlen_2021.html (accessed on 7 April 2022).
- Statistisches Bundesamt. Bevölkerungsstand: Amtliche Einwohnerzahl Deutschlands. 2022. Available online: https://www.destatis.de/DE/Themen/Gesellschaft-Umwelt/Bevoelkerung/Bevoelkerungsstand/_inhalt.html (accessed on 7 April 2022).
- Statista. Sterbeziffern Nach Alter Und Geschlecht in Deutschland. 2020. Available online: https://de.statista.com/statistik/daten/studie/3057/umfrage/sterbeziffern-nach-alter-und-geschlecht/ (accessed on 7 April 2022).
- Demografieportal. Altersstruktur Der Bevölkerung. Available online: https://www.demografie-portal.de/DE/Fakten/bevoelkerung-altersstruktur.html (accessed on 7 April 2022).
- Eurostat. GDP per Capita. Available online: https://ec.europa.eu/eurostat/databrowser/view/sdg_10_10/default/table (accessed on 10 April 2022).
- Schilling, J.; Lehfeld, A.-S.; Schumacher, D.; Diercke, M.; Buda, S.; Haas, W. Krankheitsschwere Der Ersten COVID-19-Welle in Deutschland Basierend Auf Den Meldungen Gemäß Infektionsschutzgesetz. J. Health Monit. 2020, 2–20. [Google Scholar] [CrossRef]
- IGES Institut GmbH. Pandemie Monitor: Die Corona-Krise Besser Verstehen. Available online: https://www.iges.com/corona/ (accessed on 16 April 2022).
- D’ardes, D.; Pontolillo, M.; Esposito, L.; Masciarelli, M.; Boccatonda, A.; Rossi, I.; Bucci, M.; Guagnano, M.T.; Ucciferri, C.; Santilli, F.; et al. Duration of COVID-19: Data from an Italian Cohort and Potential Role for Steroids. Microorganisms 2020, 8, 1327. [Google Scholar] [CrossRef]
- Ma, Q.; Liu, J.; Liu, Q.; Kang, L.; Liu, R.; Jing, W.; Wu, Y.; Liu, M. Global Percentage of Asymptomatic SARS-CoV-2 Infections Among the Tested Population and Individuals With Confirmed COVID-19 Diagnosis: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2021, 4, e2137257. [Google Scholar] [CrossRef]
- Bundesministerium für Gesundheit. Coronavirus SARS-CoV-2 Informationen Und Praktische Hinweise. 11 May 2021. Available online: https://www.bundesgesundheitsministerium.de/fileadmin/Dateien/3_Downloads/C/Coronavirus/Publikationen/BMG_Coronavirus_Information_Deutsch.pdf (accessed on 10 April 2022).
- Robert Koch-Institut. Coronavirus SARS-CoV-2—COVID-19-Fälle Nach Altersgruppe Und Meldewoche. Available online: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Daten/Altersverteilung.html (accessed on 10 April 2022).
- Bi, Q.; Wu, Y.; Mei, S.; Ye, C.; Zou, X.; Zhang, Z.; Liu, X.; Wei, L.; Truelove, S.A.; Zhang, T.; et al. Epidemiology and Transmission of COVID-19 in 391 Cases and 1286 of Their Close Contacts in Shenzhen, China: A Retrospective Cohort Study. Lancet Infect Dis. 2020, 20, 911–919. [Google Scholar] [CrossRef]
- Ibitoye, O.S.; Olasunkanmi, Y.A.; Olowolafe, T.A.; Olabode, A.T.; Salawu, M.M.; Afolabi, R.F. Predictors and Time to Recovery from COVID-19 among Patients Attended at the Treatment Centers in Ekiti State, South West, Nigeria. PAMJ. 2022, 42, 18. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Lorin, E. Estimation of COVID-19 Recovery and Decease Periods in Canada Using Delay Model. Sci. Rep. 2021, 11, 23763. [Google Scholar] [CrossRef] [PubMed]
- Olsen, J. Corona-Patienten: Was Kostet Die Behandlung? 25 January 2022. Available online: https://www.mdr.de/nachrichten/deutschland/panorama/corona-behandlung-kosten-100.html (accessed on 10 April 2022).
- Robert Koch-Institut. COVID-19-Hospitalisierungen in Deutschland. Available online: https://github.com/robert-koch-institut/COVID-19-Hospitalisierungen_in_Deutschland/blob/master/Aktuell_Deutschland_COVID-19-Hospitalisierungen.csv (accessed on 10 April 2022).
- Deutsche Interdisziplinäre Vereinigung für Intensiv- und Notfallmedizin e. V. DIVI Intensivregister. Available online: https://www.intensivregister.de/#/aktuelle-lage/zeitreihen (accessed on 10 April 2022).
- Schulz, K. Deutsche Rentenversicherung Post-COVID-Rehabilitation. Available online: https://www.deutsche-rentenversicherung.de/Bund/DE/Presse/Presseseminare/2021_06_29_30_berlin/06_29_praesentation_schultz.pdf (accessed on 18 April 2022).
- AfD-Fraktion im deutschen Bundestag. Cotar: Regierung Blamiert Sich Mit Der Corona-Warn-App. 24 July 2020. Available online: https://afdbundestag.de/cotar-regierung-blamiert-sich-mit-der-corona-warn-app/ (accessed on 15 April 2022).
- Lauring, A.S.; Tenforde, M.W.; Chappell, J.D.; Gaglani, M.; Ginde, A.A.; McNeal, T.; Ghamande, S.; Douin, D.J.; Talbot, H.K.; Casey, J.D.; et al. Clinical Severity of, and Effectiveness of MRNA Vaccines against, COVID-19 from Omicron, Delta, and Alpha SARS-CoV-2 Variants in the United States: Prospective Observational Study. BMJ 2022, 376, e069761. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, A.; McMenamin, J.; Taylor, B.; Robertson, C. SARS-CoV-2 Delta VOC in Scotland: Demographics, Risk of Hospital Admission, and Vaccine Effectiveness. Lancet 2021, 397, 2461–2462. [Google Scholar] [CrossRef]
- Twohig, K.A.; Nyberg, T.; Zaidi, A.; Thelwall, S.; Sinnathamby, M.A.; Aliabadi, S.; Seaman, S.R.; Harris, R.J.; Hope, R.; Lopez-Bernal, J.; et al. Hospital Admission and Emergency Care Attendance Risk for SARS-CoV-2 Delta (B.1.617.2) Compared with Alpha (B.1.1.7) Variants of Concern: A Cohort Study. Lancet Infect Dis. 2022, 22, 35–42. [Google Scholar] [CrossRef]
- Taylor, C.A.; Patel, K.; Pham, H.; Whitaker, M.; Anglin, O.; Kambhampati, A.K.; Milucky, J.; Chai, S.J.; Kirley, P.D.; Alden, N.B.; et al. Severity of Disease Among Adults Hospitalized with Laboratory-Confirmed COVID-19 Before and During the Period of SARS-CoV-2 B.1.617.2 (Delta) Predominance—COVID-NET, 14 States, January-August 2021. MMWR Morb. Mortal. Wkly Rep. 2021, 70, 1513–1519. [Google Scholar] [CrossRef]
- Nyberg, T.; Ferguson, N.M.; Nash, S.G.; Webster, H.H.; Flaxman, S.; Andrews, N.; Hinsley, W.; Bernal, J.L.; Kall, M.; Bhatt, S.; et al. Comparative Analysis of the Risks of Hospitalisation and Death Associated with SARS-CoV-2 Omicron (B.1.1.529) and Delta (B.1.617.2) Variants in England: A Cohort Study. Lancet 2022, 399, 1303–1312. [Google Scholar] [CrossRef]
- Riley, S.; Fraser, C.; Donnelly, C.A.; Ghani, A.C.; Abu-Raddad, L.J.; Hedley, A.J.; Leung, G.M.; Ho, L.M.; Lam, T.H.; Thach, T.Q.; et al. Transmission Dynamics of the Etiological Agent of SARS in Hong Kong: Impact of Public Health Interventions. Science 2003, 300, 1961–1966. [Google Scholar] [CrossRef] [Green Version]
- Kwok, K.O.; Tang, A.; Wei, V.W.I.; Park, W.H.; Yeoh, E.K.; Riley, S. Epidemic Models of Contact Tracing: Systematic Review of Transmission Studies of Severe Acute Respiratory Syndrome and Middle East Respiratory Syndrome. Comput. Struct. Biotechnol. J. 2019, 17, 186–194. [Google Scholar] [CrossRef]
- Li, H.; Zhu, Y.; Niu, Y.; Li, H.; Zhu, Y.; Niu, Y. Contact Tracing Research: A Literature Review Based on Scientific Collaboration Network. Int. J. Environ. Res. Public Health 2022, 19, 9311. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.; Cowls, J.; Taddeo, M.; Floridi, L. Ethical Guidelines for COVID-19 Tracing Apps. Nature 2020, 582, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Afroogh, S.; Esmalian, A.; Mostafavi, A.; Akbari, A.; Rasoulkhani, K.; Esmaeili, S.; Hajiramezanali, E. Tracing App Technology: An Ethical Review in the COVID-19 Era and Directions for Post-COVID-19. Ethics Inf. Technol. 2022, 24, 30. [Google Scholar] [CrossRef]
- Nabeel, A.; Al-Sabah, S.K.; Ashrafian, H. Digital Contact Tracing Applications against COVID-19: A Systematic Review. Med. Princ. Pract. 2022. [Google Scholar] [CrossRef] [PubMed]
- Felipe, L.; Ramos, M. Evaluating Privacy during the COVID-19 Public Health Emergency: The Case of Facial Recognition Technologies. In Proceedings of the 13th International Conference on Theory and Practice of Electronic Governance, New York, NY, USA, 29 October 2020; Volume 2020, pp. 23–25. [Google Scholar] [CrossRef]
- Pratt, B.; Parker, M.; Bull, S. Equitable Design and Use of Digital Surveillance Technologies During COVID-19: Norms and Concerns. J. Empir. Res. Hum. Res. Ethics 2022. [Google Scholar] [CrossRef]
- Nunes, N.; Adamo, G.; Ribeiro, M.; Gouveia, B.R.; Rubio Gouveia, E.; Teixeira, P.; Nisi, V. Modeling Adoption, Security, and Privacy of COVID-19 Apps: Findings and Recommendations From an Empirical Study Using the Unified Theory of Acceptance and Use of Technology. JMIR Hum. Factors 2022, 9, e35434. [Google Scholar] [CrossRef]
- Bardus, M.; al Daccache, M.; Maalouf, N.; al Sarih, R.; Elhajj, I.H. Data Management and Privacy Policy of COVID-19 Contact-Tracing Apps: Systematic Review and Content Analysis. JMIR Mhealth Uhealth 2022, 10, e35195. [Google Scholar] [CrossRef]
- Tomczyk, S. Appsolutely Secure? Psychometric Properties of the German Version of an App Information Privacy Concerns Measure during COVID-19. Front. Psychol. 2022, 13, 899092. [Google Scholar] [CrossRef]
- Hatke, G.F.; Montanari, M.; Appadwedula, S.; Wentz, M.; Meklenburg, J.; Ivers, L.; Watson, J.; Fiore, P. Using Bluetooth Low Energy (BLE) Signal Strength Estimation to Facilitate Contact Tracing for COVID-19. arXiv 2020, arXiv:2006.15711. [Google Scholar] [CrossRef]
- Shahroz, M.; Ahmad, F.; Younis, M.S.; Ahmad, N.; Kamel Boulos, M.N.; Vinuesa, R.; Qadir, J. COVID-19 Digital Contact Tracing Applications and Techniques: A Review Post Initial Deployments. Transp. Eng. 2021, 5, 100072. [Google Scholar] [CrossRef]
- Cranor, L.F. Digital Contact Tracing May Protect Privacy, but It Is Unlikely to Stop the Pandemic. Commun. ACM 2020, 63, 22–24. [Google Scholar] [CrossRef]
- Loh, P.-S. Accuracy of Bluetooth-Ultrasound Contact Tracing: Experimental Results from NOVID IOS Version 2.1 Using Five-Year-Old Phones. NOVID 2020. [Google Scholar]
- Hasan, H.R.; Salah, K.; Jayaraman, R.; Yaqoob, I.; Omar, M.; Ellahham, S. COVID-19 Contact Tracing Using Blockchain. IEEE Access 2021, 9, 62956–62971. [Google Scholar] [CrossRef]
- Jahmunah, V.; Sudarshan, V.K.; Oh, S.L.; Gururajan, R.; Gururajan, R.; Zhou, X.; Tao, X.; Faust, O.; Ciaccio, E.J.; Ng, K.H.; et al. Future IoT Tools for COVID-19 Contact Tracing and Prediction: A Review of the State-of-the-Science. Int. J. Imaging Syst. Technol. 2021, 31, 455–471. [Google Scholar] [CrossRef]
- Leith, D.J.; Farrell, S. Measurement-Based Evaluation of Google/Apple Exposure Notification API for Proximity Detection in a Light-Rail Tram. PLoS ONE 2020, 15, e0239943. [Google Scholar] [CrossRef] [PubMed]
- Amann, J.; Sleigh, J.; Vayena, E. Digital Contact-Tracing during the COVID-19 Pandemic: An Analysis of Newspaper Coverage in Germany, Austria, and Switzerland. PLoS ONE 2021, 16, e0246524. [Google Scholar] [CrossRef]
- Leung, K.K.; Zhang, R.; Hashim, M.J.; Fang, M.; Xu, J.; Sun, D.; Li, X.; Liu, Y.; Deng, H.; Zeng, D.; et al. Effectiveness of Containment Strategies in Preventing SARS-CoV-2 Transmission. J. Infect. Public Health 2022, 15, 609. [Google Scholar] [CrossRef] [PubMed]
- Yeo, V.A.; Mi, Y.; Kwok, K.T. Factors Affecting Adoption of Digital Contact Tracing during the COVID-19 Pandemic: A Literature Review. J. Public Health Emerg. 2022, 6, 23. [Google Scholar] [CrossRef]
- Chen, A.T.-Y.; Thio, K.W. Exploring the Drivers and Barriers to Uptake for Digital Contact Tracing. Soc. Sci. Humanit. Open 2021, 4, 100212. [Google Scholar] [CrossRef]
- Dzandu, M.D.; Pathak, B.; de Cesare, S. Acceptability of the COVID-19 Contact-Tracing App—Does Culture Matter? Gov. Inf. Q 2022, 39, 101750. [Google Scholar] [CrossRef]
- Horstmann, K.T.; Buecker, S.; Krasko, J.; Kritzler, S.; Terwiel, S. Who Does or Does Not Use the “Corona-Warn-App” and Why? Eur. J. Public Health 2021, 31, 49–51. [Google Scholar] [CrossRef] [PubMed]
- Grekousis, G.; Liu, Y. Digital Contact Tracing, Community Uptake, and Proximity Awareness Technology to Fight COVID-19: A Systematic Review. Sustain. Cities Soc. 2021, 71, 102995. [Google Scholar] [CrossRef] [PubMed]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-Acute COVID-19 Syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.D.; Lavelle, M.; Boursiquot, B.C.; Wan, E.Y. Long-Term Complications of COVID-19. Am. J. Physiol. Cell Physiol. 2022, 322, C1–C11. [Google Scholar] [CrossRef] [PubMed]
- Crook, H.; Raza, S.; Nowell, J.; Young, M.; Edison, P. Long COVID-Mechanisms, Risk Factors, and Management. BMJ 2021, 374, n1648. [Google Scholar] [CrossRef]
- Antonelli, M.; Penfold, R.S.; Merino, J.; Sudre, C.H.; Molteni, E.; Berry, S.; Canas, L.S.; Graham, M.S.; Klaser, K.; Modat, M.; et al. Risk Factors and Disease Profile of Post-Vaccination SARS-CoV-2 Infection in UK Users of the COVID Symptom Study App: A Prospective, Community-Based, Nested, Case-Control Study. Lancet Infect. Dis. 2022, 22, 43–55. [Google Scholar] [CrossRef]
- Broughel, J.; Kotrous, M. The Benefits of Coronavirus Suppression: A Cost-Benefit Analysis of the Response to the First Wave of COVID-19 in the United States. PLoS ONE 2021, 16, e0252729. [Google Scholar] [CrossRef]
- Doti, J. Benefit-Cost Analysis of COVID-19 Policy Intervention at the State and National Level. Econ. Fac. Artic. Res. 2021. Available online: https://digitalcommons.chapman.edu/economics_articles/257 (accessed on 19 April 2022).
- Endt, C. Corona-Inzidenz: Diskussionen Um Die Schwellenwerte 50 Und 35. 17 January 2021. Available online: https://www.sueddeutsche.de/politik/corona-inzidenz-schwellenwerte-50-35-1.5208343 (accessed on 19 April 2022).
- Harrington, W.N.; Kackos, C.M.; Webby, R.J. The Evolution and Future of Influenza Pandemic Preparedness. Exp. Mol. Med. 2021, 53, 737–749. [Google Scholar] [CrossRef]
- Watts, G. COVID-19 and the Digital Divide in the UK. Lancet Digit Health 2020, 2, e395–e396. [Google Scholar] [CrossRef]
- Grabowski, F.; Kochańczyk, M.; Lipniacki, T. The Spread of SARS-CoV-2 Variant Omicron with a Doubling Time of 2.0–3.3 Days Can Be Explained by Immune Evasion. Viruses 2022, 14, 294. [Google Scholar] [CrossRef] [PubMed]
- Hart, W.S.; Miller, E.; Andrews, N.J.; Waight, P.; Maini, P.K.; Funk, S.; Thompson, R.N. Generation Time of the Alpha and Delta SARS-CoV-2 Variants: An Epidemiological Analysis. Lancet Infect. Dis. 2022, 22, 603–610. [Google Scholar] [CrossRef]
- Dichmann, M.; Banse, P. Corona-Warn-App: Positiven Corona-Test Teilen Ist Vielen Zu Kompliziert. 30 October 2020. Available online: https://www.deutschlandfunknova.de/beitrag/corona-warn-app-positiven-corona-test-teilen-ist-vielen-zu-kompliziert (accessed on 19 April 2022).
- Walkey, A.J.; Law, A.; Bosch, N.A. Lottery-Based Incentive in Ohio and COVID-19 Vaccination Rates. JAMA 2021, 326, 766–767. [Google Scholar] [CrossRef]
- Shubina, V.; Ometov, A.; Basiri, A.; Lohan, E.S. Effectiveness Modelling of Digital Contact-Tracing Solutions for Tackling the COVID-19 Pandemic. J. Navig. 2021, 74, 1. [Google Scholar] [CrossRef]
- Meier, Y.; Meinert, J.; Krämer, N.C. Investigating Factors That Affect the Adoption of COVID-19 Contact-Tracing Apps: A Privacy Calculus Perspective. Technol. Mind Behav. 2021, 2, 1–10. [Google Scholar] [CrossRef]








| Adoption Rate | Reduction in Reff | Reff/Rhyp in Case of Rhyp = 1 | Reference |
|---|---|---|---|
| 20% | 17.6% | 0.824 | Kretzschmar et al. [10] |
| 40% | 20.2% | 0.798 | |
| 60% | 24.4% | 0.756 | |
| 80% | 30.4% | 0.696 | |
| 100% | 38.1% | 0.619 | |
| 53% | 47% | 0.53 | Kucharski et al. [11] |
| 40% | 38.7% | 0.613 | Plank et al. [12] |
| 60% | 40.8% | 0.592 | |
| 80% | 41.7% | 0.583 | |
| 40% | 33.3% | 0.667 | Elmokashfi et al. [13] 1 |
| Date | Costs, EUR | Benefits, EUR | NPV, EUR | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| App | Tests | Pensions | Sum | IfW | HO | IC | Death | Rehab | Sum | ||
| 20 July | 27 | 0 | 0 | 27 | 0 | 0 | 0 | 0 | 0 | 0 | −27 |
| 20 August | 33 | 0 | 0 | 33 | 0 | 0 | 0 | 0 | 0 | 1 | −33 |
| 20 September | 39 | 1 | 0 | 40 | 0 | 0 | 0 | 0 | 0 | 0 | −40 |
| 20 October | 45 | 2 | 0 | 47 | 2 | 0 | 0 | 0 | 0 | 2 | −45 |
| 20 November | 52 | 5 | 0 | 56 | −5 | −1 | −1 | 0 | −1 | −7 | −64 |
| 20 December | 58 | 12 | 2 | 72 | 10 | 2 | 3 | 1 | 2 | 18 | −54 |
| 21 January | 64 | 20 | 13 | 97 | 40 | 10 | 13 | 5 | 10 | 78 | −19 |
| 21 February | 70 | 27 | 27 | 124 | 68 | 17 | 24 | 10 | 18 | 137 | 13 |
| 21 March | 76 | 30 | 36 | 141 | 84 | 23 | 32 | 13 | 24 | 175 | 33 |
| 21 April | 82 | 34 | 38 | 155 | 93 | 25 | 34 | 14 | 26 | 191 | 36 |
| 21 May | 88 | 44 | 41 | 173 | 124 | 30 | 42 | 15 | 31 | 242 | 69 |
| 21 June | 94 | 51 | 44 | 188 | 147 | 35 | 48 | 16 | 36 | 281 | 93 |
| 21 July | 100 | 51 | 45 | 196 | 151 | 36 | 50 | 16 | 37 | 289 | 93 |
| 21 August | 106 | 53 | 45 | 204 | 153 | 36 | 50 | 16 | 38 | 293 | 89 |
| 21 September | 112 | 55 | 45 | 213 | 156 | 36 | 50 | 16 | 38 | 297 | 84 |
| 21 October | 118 | 60 | 46 | 224 | 180 | 41 | 54 | 16 | 42 | 333 | 109 |
| 21 November | 124 | 75 | 47 | 246 | 204 | 45 | 58 | 17 | 45 | 368 | 123 |
| 21 December | 130 | 147 | 48 | 325 | 261 | 51 | 64 | 17 | 51 | 444 | 118 |
| 22 January | 134 | 215 | 51 | 400 | 442 | 69 | 85 | 18 | 69 | 683 | 283 |
| 22 February | 137 | 474 | 53 | 664 | 600 | 75 | 90 | 19 | 73 | 856 | 192 |
| 22 March | 141 | 826 | 53 | 1020 | 1156 | 92 | 102 | 19 | 88 | 1457 | 437 |
| 22 April | 144 | 1436 | 54 | 1634 | 2040 | 114 | 120 | 19 | 106 | 2399 | 765 |
| Research Topic | DCT Apps in General | CWA-Specific |
|---|---|---|
| Privacy, security, ethics | Afroogh et al. [59] Morley et al. [58] Nabeel et al. [60] Felipe at al. [61] Pratt et al. [62] Nunes et al. [63] Bardus et al. [64] | Afroogh et al. [59] Morley et al. [58] Tomczyk [65] |
| Technical issues | Ferretti et al. [2] Hatke et al. [66] Shahroz et al. [67] Cranor et al. [68] Loh et al. [69] Felipe at al. [61] Jahmunah et al. [71] Hasan et al. [70] | n/a |
| Sociodemographic studies | Yeo at al. [75] Chen et al. [76] Dzandu et al. [77] | Grill et al. [8] Amann et al. [73] Horstmann et al. [78] Munzert et al. [6] |
| Impact of DCT apps on R-value | Jenniskens et al. [17] Kretzschmar et al. [10] Kucharski et al. [11] Plank et al. [12] Elmokashfi et al. [13] | n/a |
| Impact of DCT apps on absolute caseloads | Leung et al. [74]1 | Leung et al. [74] 1 |
| Cost–Benefit studies | n/a | n/a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ellmann, S.; Maryschok, M.; Schöffski, O.; Emmert, M. The German COVID-19 Digital Contact Tracing App: A Socioeconomic Evaluation. Int. J. Environ. Res. Public Health 2022, 19, 14318. https://doi.org/10.3390/ijerph192114318
Ellmann S, Maryschok M, Schöffski O, Emmert M. The German COVID-19 Digital Contact Tracing App: A Socioeconomic Evaluation. International Journal of Environmental Research and Public Health. 2022; 19(21):14318. https://doi.org/10.3390/ijerph192114318
Chicago/Turabian StyleEllmann, Stephan, Markus Maryschok, Oliver Schöffski, and Martin Emmert. 2022. "The German COVID-19 Digital Contact Tracing App: A Socioeconomic Evaluation" International Journal of Environmental Research and Public Health 19, no. 21: 14318. https://doi.org/10.3390/ijerph192114318
APA StyleEllmann, S., Maryschok, M., Schöffski, O., & Emmert, M. (2022). The German COVID-19 Digital Contact Tracing App: A Socioeconomic Evaluation. International Journal of Environmental Research and Public Health, 19(21), 14318. https://doi.org/10.3390/ijerph192114318