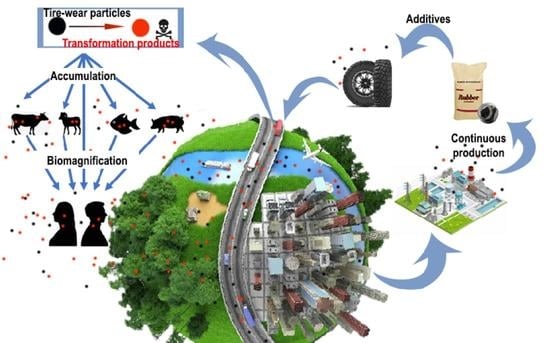
Rubber Antioxidants and Their Transformation Products: Environmental Occurrence and Potential Impact
Abstract
:1. Introduction
2. Production and Use of Typical Rubber Antioxidants
2.1. Amine Antioxidants
2.2. Phenolic Antioxidants
2.3. Heterocyclic Antioxidants
2.4. Phosphite Antioxidants
3. Formation Mechanism of TPs in the Environment
4. Toxic Effects of Antioxidants and TPs
5. Environmental Occurrence and Potential Impact of Antioxidants and TPs
| Environmental Medium | Compound | Sampling Location | Concentrations | References |
|---|---|---|---|---|
| Water (µg/L) | DPG | Urban streams (Canada) | 0.76 ± 0.05 | [71] |
| WWTP Discharge (Canada) | 0.06 ± 0.01 | |||
| Seattle-area waterways (America) | 0.02 | [84] | ||
| Regional center (Queensland, Australia) | <0.1 | [58,66] | ||
| Brisbane (Queensland, Australia) | 0.05–1.08 | |||
| 6PPD-Q | The influent of WWTP treating wastewater in the snow-melt day (Leipzig, Germany) | 0.11 ± 0.04 | [40] | |
| Urban streams (Canada) | 0.72 ± 0.26 | [71] | ||
| Near WWTP Discharge (Canada) | 0.05 ± 0.02 | |||
| Surface water (Michigan, America) | <0.04 | [69] | ||
| Standing road water (Michigan, America) | 0.05–0.66 | |||
| Regional center (Queensland, Australia) | <0.02 | [58,66] | ||
| Brisbane (Queensland, Australia) | <0.09 | |||
| Urban river in the Don River (Canada) | 2.30 ± 0.05 | [85] | ||
| Runoff water (Hong Kong, China) | 0.21–2.43 | [73] | ||
| HMMM | Urban streams (Canada) | 2.26 ± 0.34 | [71] | |
| Surface water in the Don River and Highland Creek (Canada) | >1 | [67] | ||
| Regional center (Queensland, Australia) | <0.29 | [58,66] | ||
| Brisbane (Queensland, Australia) | 0.01–0.20 | |||
| HMMM | German rivers (Germany) | 0.01–0.88 | [86] | |
| Water treatment plants, influent (Southern Ontario, Canada) | <0.01–0.03 | [68] | ||
| Water treatment plants, effluent (Southern Ontario, Canada) | 0.02–0.11 | |||
| HMMM TPs | Urban streams (Canada) | <11.2 | [71] | |
| Dust (ng/g) | 6PPD | Road (Tokyo, Japan) | 45–1175 | [39] |
| Road (Guangzhou, China) | 4.1–238 | [75] | ||
| Parking lot (Guangzhou, China) | 13.5–429 | |||
| Vehicle (Guangzhou, China) | 5.0–41.9 | |||
| House (Guangzhou, China) | n.d.a–6.1 | |||
| Roadside soils (Hong Kong, China) | 31.4–831 | [73] | ||
| Indoor dust (Beijing) | n.d.–0.28 | [87] | ||
| Playground dust (Beijing) | n.d.–0.69 | |||
| 6PPD-Q | Road (Tokyo, Japan) | 870–8520 | [39] | |
| Road (Guangzhou, China) | 32.2–80.9 | [75] | ||
| Parking lot (Guangzhou, China) | 5.7–277 | |||
| Vehicle (Guangzhou, China) | 17.9–146 | |||
| House (Guangzhou, China) | n.d.–0.4 | |||
| Roadside soils (Hong Kong, China) | 9.50–936 | [73] | ||
| E-waste recycling workshops (south China) | 375 | [88] | ||
| 77PD | Road (Guangzhou, China) | n.d.–38.5 | [75] | |
| Parking lot (Guangzhou, China) | n.d.–29.1 | |||
| Vehicle (Guangzhou, China) | n.d.–9.6 | |||
| House (Guangzhou, China) | n.d.–77.6 | |||
| DNPD | Road (Guangzhou, China) | 1.5–35.9 | [75] | |
| Parking lot (Guangzhou, China) | n.d.–28.9 | |||
| Vehicle (Guangzhou, China) | 1.9–29.5 | |||
| House (Guangzhou, China) | n.d.–137 | |||
| CPPD | Road (Guangzhou, China) | 3.4–190 | [75] | |
| Parking lot (Guangzhou, China) | 5.8–540 | |||
| Vehicle (Guangzhou, China) | 5.2–66.8 | |||
| House (Guangzhou, China) | n.d.–0.4 | |||
| Roadside soils (Hong Kong, China) | 0.73–15.4 | [73] | ||
| DPPD | Road (Guangzhou, China) | 5.8–126 | [75] | |
| Parking lot (Guangzhou, China) | 16.4–217 | |||
| Vehicle (Guangzhou, China) | n.d.–55.3 | |||
| House (Guangzhou, China) | n.d.–27.0 | |||
| Roadside soils (Hong Kong, China) | 3.63–84.4 | [73] | ||
| Indoor dust (Beijing) | n.d.–22.2 | [87] | ||
| Playground dust in Beijing | n.d.–22.6 | |||
| IPPD | Road (Guangzhou, China) | n.d.–321 | [75] | |
| Parking lot (Guangzhou, China) | n.d.–237 | |||
| Vehicle (Guangzhou, China) | n.d.–575 | |||
| House (Guangzhou, China) | n.d.–41.5 | |||
| Roadside soils (Hong Kong, China) | 0.66–24.5 | [73] | ||
| E-waste recycling workshops (south China) | 363 | [88] | ||
| Air (pg/m3) | IPPD | Hong Kong Baptist University (Hong Kong, China) | 0.44–2.73 | [73] |
| Shanxi University (Taiyuan, China) | 0.3–8.3 | [72] | ||
| Zhengzhou University (Zhengzhou, China) | 0.3–50 | |||
| Fudan University (Shanghai, China) | 0.3–104 | |||
| Jiangsu Provincial Center for Disease Control and Prevention (Nanjing, China) | 0.8–4.7 | |||
| Government of Hangzhou Binjiang District (Hangzhou, China) | 0.4–2.4 | |||
| Guangdong University of Technology (Guangzhou, China) | 0.2–5.7 | |||
| DPPD | Hong Kong Baptist University (Hong Kong, China) | n.d.–0.70 | [73] | |
| Shanxi University (Taiyuan, China) | 0.1–8.2 | [72] | ||
| Zhengzhou University (Zhengzhou, China) | 0.1–1.5 | |||
| Fudan University (Shanghai, China) | 0.1–5.6 | |||
| Jiangsu Provincial Center for Disease Control and Prevention (Nanjing, China) | 0.1–13 | |||
| Government of Hangzhou Binjiang District (Hangzhou, China) | 0.1–5.8 | [72] | ||
| Guangdong University of Technology (Guangzhou, China) | 0.1–1 | |||
| CPPD | Hong Kong Baptist University (Hong Kong, China) | n.d.–0.74 | [73] | |
| Shanxi University (Taiyuan, China) | 0.5–14 | [72] | ||
| Zhengzhou University (Zhengzhou, China) | 0.4–4.2 | |||
| Fudan University (Shanghai, China) | 0.4–21 | |||
| Jiangsu Provincial Center for Disease Control and Prevention (Nanjing, China) | 0.3–1.2 | |||
| Government of Hangzhou Binjiang District (Hangzhou, China) | 0.4–3.0 | |||
| Guangdong University of Technology (Guangzhou, China) | 0.1–5.1 | |||
| 6PPD | Hong Kong Baptist University (Hong Kong, China) | 0.82–6.30 | [73] | |
| Shanxi University (Taiyuan, China) | 0.02–487 | [72] | ||
| Zhengzhou University (Zhengzhou, China) | 1.2–109 | |||
| Fudan University (Shanghai, China) | 0.5–135 | |||
| Jiangsu Provincial Center for Disease Control and Prevention (Nanjing, China) | 0.4–75 | |||
| Government of Hangzhou Binjiang District (Hangzhou, China) | 0.1–6.0 | |||
| Guangdong University of Technology (Guangzhou, China) | 0.3–10 | |||
| DNPD | Shanxi University (Taiyuan, China) | 0.5–14 | [72] | |
| Zhengzhou University (Zhengzhou, China) | 0.6–7.1 | |||
| Fudan University (Shanghai, China) | 0.5–108 | |||
| Jiangsu Provincial Center for Disease Control and Prevention (Nanjing, China) | 0.3–4.7 | |||
| Government of Hangzhou Binjiang District (Hangzhou, China) | 1.4–9.9 | [72] | ||
| Guangdong University of Technology (Guangzhou, China) | 0.5–5.5 | |||
| 77PD | Shanxi University (Taiyuan, China) | 0.2–7052 | [72] | |
| Zhengzhou University (Zhengzhou, China) | 0.5–231 | |||
| Fudan University (Shanghai, China) | 0.05–967 | |||
| Jiangsu Provincial Center for Disease Control and Prevention (Nanjing, China) | 0.1–84 | |||
| Government of Hangzhou Binjiang District (Hangzhou, China) | 0.5–93 | |||
| Guangdong University of Technology (Guangzhou, China) | 0.1–693 | |||
| 6PPD-Q | Hong Kong Baptist University (Hong Kong, China) | 0.54–13.8 | [73] |
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- ASTM D1566-21a; Standard Terminology Relating to Rubber. ASTM International: West Conshohocken, PA, USA, 2020. [CrossRef]
- Pan, Z.D.; Ding, Y.J.; Yan, L.; Li, X.M.; Jiao, G.P.; Luo, H.Y. Study on copper-based catalysts for synthesis of N,N’-bis(1,4-dimethylpentyl)-p-phenylenediamine from reductive alkylation of p-Nitroaniline with 5-methyl-2-hexanone. Catal. Lett. 2008, 122, 115–120. [Google Scholar] [CrossRef]
- Sarkar, P.; Bhowmick, A.K. Sustainable rubbers and rubber additives. J. Appl. Polym. Sci. 2017, 135, 45701. [Google Scholar] [CrossRef] [Green Version]
- Öncel, Ş.; Kurtoğlu, B.; Karaağaç, B. An alternative antioxidant for sulfur-vulcanized natural rubber. J. Elastomers Plast. 2018, 51, 440–456. [Google Scholar] [CrossRef]
- Pike, M.; Watson, W.F. Mastication of rubber mechanism of plasticizing by cold mastication. J. Polym. Sci. 2010, 9, 229–251. [Google Scholar] [CrossRef]
- Maher, B.M.; Rezaali, J.; Jalili, K.; Abbasi, F. Effects of various treatments on silicone rubber surface. Rubber Chem. Technol. 2017, 90, 108–125. [Google Scholar] [CrossRef]
- Tian, Z.Y.; Zhao, H.; Peter, K.T.; Gonzalez, M.; Wetzel, J.; Wu, C.; Hu, X.; Prat, J.; Mudrock, E.; Hettinger, R. A ubiquitous tire rubber–derived chemical induces acute mortality in coho salmon. Science 2020, 371, 185–189. [Google Scholar] [CrossRef]
- Men, Z.Y.; Zhang, X.F.; Peng, J.F.; Zhang, J.; Fang, T.G.; Guo, Q.Y.; Wei, N.; Zhang, Q.J.; Wang, T.; Wu, L.; et al. Determining factors and parameterization of brake wear particle emission. J. Hazard. Mater. 2022, 434, 128856. [Google Scholar] [CrossRef]
- Ding, J.; Zhu, D.; Wang, H.T.; Lassen, S.B.; Zhu, Y.G. Dysbiosis in the gut microbiota of soil fauna explains the toxicity of tire tread particles. Environ. Sci. Technol. 2020, 54, 7450–7460. [Google Scholar] [CrossRef]
- Guo, X.H.; Luo, Y.F.; Chen, L.J.; Zhang, B.W.; Chen, Y.J.; Jia, D.M. Biomass antioxidant silica supported tea polyphenols with green and high-efficiency free radical capturing activity for rubber composites. Compos. Sci. Technol. 2022, 220, 109290. [Google Scholar] [CrossRef]
- Zhang, X. Analysis of the Current Market Situation of Phosphorous Flame Retardants Industry in China in 2020 Shows Significant Market and Performance Advantages. Available online: https://www.huaon.com/channel/trend/741225.html (accessed on 20 April 2022).
- Dai, M.X.; Liang, C. Current situation and market analysis of rubber antioxidant industry. Econ. Anal. China Pet. Chem. Ind. 2014, 7, 3. [Google Scholar]
- Association, C.R.I. Statistical Analysis of China’s Rubber Additives Industry in 2020. Available online: http://www.rubberchem.com.cn/info/202132/20213295208.shtml (accessed on 27 January 2022).
- Abad, L.V.; Relleve, L.S.; Aranilla, C.T.; Aliganga, A.K.; San Diego, C.M.; dela Rosa, A.M. Natural antioxidants for radiation vulcanization of natural rubber latex. Polym. Degrad. Stab. 2002, 76, 275–279. [Google Scholar] [CrossRef]
- Zheng, T.; Zheng, X.; Zhan, S.; Zhou, J.; Liao, S. Study on the ozone aging mechanism of natural rubber. Polym. Degrad. Stab. 2021, 186, 109514. [Google Scholar] [CrossRef]
- Li, G.-Y.; Koenig, J.L. A review of rubber oxidation. Rubber Chem. Technol. 2005, 78, 355–390. [Google Scholar] [CrossRef]
- Lu, N.; Shen, M.; Hou, Z.; Prakashan, K.; Xin, Z. Effectiveness of different kinds of antioxidants in resin-cured bromobutyl rubber vulcanizates. Adv. Polym. Technol. 2017, 37, 2075–2084. [Google Scholar] [CrossRef]
- Bravar, M.; Rolich, J.; Biga, N. Protection of natural rubber films against thermal ageing by addition of amine and phenolic anti-oxidants. Eur. Polym. J. 1980, 16, 637–640. [Google Scholar] [CrossRef]
- Sahakaro, K.; Naskar, N.; Datta, R.N.; Noordermeer, J.W.M. Reactive blending, reinforcement and curing of NR/BR/EPDM compounds for tire sidewall applications. Rubber Chem. Technol. 2007, 80, 115–138. [Google Scholar] [CrossRef]
- Zeb, A. Applications of phenolic antioxidants. In Phenolic Antioxidants in Foods: Chemistry, Biochemistry and Analysis; Springer International Publishing: Cham, Switzerland, 2021; pp. 385–411. [Google Scholar]
- Ahmadi, S.; Arabi, H. Enhanced thermo-oxidative stability through covalent attachment of hindered phenolic antioxidant on surface functionalized polypropylene. Polymer 2018, 138, 41–48. [Google Scholar]
- Barret, J.; Gijsman, P.; Swagten, J.; Lange, R. The interaction of a phenolic anti-oxidant and an aromatic amine in a thermo-oxidative ageing process. Polym. Degrad. Stab. 2002, 75, 367–374. [Google Scholar] [CrossRef]
- Sulekha, P.; Joseph, R.B. Preparation and characterisation of novel polymer bound phenolic antioxidants and its use in natural rubber. J. Elastomers Plast. 2003, 35, 85–97. [Google Scholar] [CrossRef]
- Herdan, J.M.; Giurginca, M. Grafting antioxidants phenols with mercaptoheterocyclic substituents as antioxidants for dienic rubbers. Polym. Degrad. Stab. 1993, 41, 157–162. [Google Scholar] [CrossRef]
- Yakout, E.M.A.; El-Sabbagh, S.H. New uracil derivatives as antioxidants for natural rubber. Pigment Resin Technol. 2007, 36, 224–234. [Google Scholar] [CrossRef]
- Hu, D.C.; Jia, Z.X.; Zhong, B.C.; Chen, Y.J.; Luo, Y.F. A facile and green preparation of nanosilica-supported antioxidant and its reinforcement and antioxidation effect on styrene-butadiene rubber. Int. J. Polym. Anal. Charact. 2016, 21, 185–197. [Google Scholar] [CrossRef]
- Wu, Y.P.; Zhang, Z.Z.; Wei, L.Q.; Yang, S.Z. A combined experimental and molecular simulation study of factors influencing the selection of antioxidants in butadiene rubber. J. Phys. Chem. B Condens. Matter Mater. Surf. Interfaces Biophys. 2017, 121, 1413–1425. [Google Scholar]
- Humphris, K.J.; Scott, G. Mechanisms of antioxidant action. Pure Appl. Chem. 1973, 36, 163–176. [Google Scholar] [CrossRef]
- Ismail, M.N.; Yehia, A.A.; Korium, A.A. Evaluation of some arylphosphites as antioxidants and antifatigue agents in natural rubber and styrene–butadiene rubber vulcanizates. Polym. Degrad. Stab. 2001, 74, 247–253. [Google Scholar] [CrossRef]
- Basfar, A.A.; Abdel-Azizl, M.M.; Mofti, S. Stabilization of γ-radiation vulcanized EPDM rubber against accelerated aging. Polym. Degrad. Stab. 1999, 66, 191–197. [Google Scholar] [CrossRef]
- Wang, W.; Cao, G.D.; Zhang, J.; Wu, P.F.; Chen, Y.Y.; Chen, Z.F.; Qi, Z.H.; Li, R.J.; Dong, C.; Cai, Z.W. Beyond substituted p-Phenylenediamine antioxidants: Prevalence of their quinone derivatives in PM2.5. Environ. Sci. Technol. 2022, 56, 10629–10637. [Google Scholar] [CrossRef]
- Flyunt, R.; Leitzke, A.; Mark, G.; Mvula, E.; Reisz, E.; Schick, R.; von Sonntag, C. Determination of •OH, O2•-, and hydroperoxide yields in ozone reactions in aqueous solution. J. Phys. Chem. B 2003, 107, 7242–7253. [Google Scholar] [CrossRef]
- Cataldo, F. A study on the reaction between N-substituted p-phenylenediamines and ozone: Experimental results and theoretical aspects in relation to their antiozonant activity. Eur. Polym. J. 2002, 38, 885–893. [Google Scholar] [CrossRef]
- Cataldo, F.; Faucette, B.; Huang, S.; Ebenezer, W. On the early reaction stages of ozone with N,N’-substituted p-phenylenediamines (6PPD, 77PD) and N,N’,N”-substituted-1,3,5-triazine “Durazone®”: An electron spin resonance (ESR) and electronic absorption spectroscopy study. Polym. Degrad. Stab. 2015, 111, 223–231. [Google Scholar] [CrossRef]
- Cataldo, F. Early stages of p-phenylenediamine antiozonants reaction with ozone: Radical cation and nitroxyl radical formation. Polym. Degrad. Stab. 2018, 147, 132–141. [Google Scholar] [CrossRef]
- Nematollahi, D.; Ghasemi, F.; Sharafi-Kolkeshvandi, M.; Varmaghani, F. Insight into the electrochemical oxidation of N,N-dialkyl-p-phenylenediamines in the presence of malononitrile and methyl cyanoacetate. A convergent paired electrochemical method for the synthesis of cyanide and dicyanide derivatives of phenylcarbonimidoyl. J. Electroanal. Chem. 2016, 775, 299–305. [Google Scholar] [CrossRef]
- Rapta, P.; Vargová, A.; Polovková, J.; Gatial, A.; Omelka, L.; Majzlík, P.; Breza, M. A variety of oxidation products of antioxidants based on N,N’-substituted p-phenylenediamines. Polym. Degrad. Stab. 2009, 94, 1457–1466. [Google Scholar] [CrossRef]
- Hu, X.M.; Zhao, H.Q.; Tian, Z.Y.; Peter, K.T.; Dodd, M.C.; Kolodziej, E.P. Transformation product formation upon heterogeneous ozonation of the tire rubber antioxidant 6PPD (N-(1,3-dimethylbutyl)-N’-phenyl-p-phenylenediamine). Environ. Sci. Technol. Lett. 2022, 9, 413–419. [Google Scholar] [CrossRef]
- Hiki, K.; Yamamoto, H. Concentration and leachability of N-(1,3-dimethylbutyl)-N’-phenyl-p-phenylenediamine (6PPD) and its quinone transformation product (6PPD-Q) in road dust collected in Tokyo, Japan. Environ. Pollut. 2022, 302, 119082. [Google Scholar] [CrossRef]
- Seiwert, B.; Nihemaiti, M.; Troussier, M.; Weyrauch, S.; Reemtsma, T. Abiotic oxidative transformation of 6-PPD and 6-PPD quinone from tires and occurrence of their products in snow from urban roads and in municipal wastewater. Water Res. 2022, 212, 118122. [Google Scholar] [CrossRef]
- Alhelou, R.; Seiwert, B.; Reemtsma, T. Hexamethoxymethylmelamine—A precursor of persistent and mobile contaminants in municipal wastewater and the water cycle. Water Res. 2019, 165, 114973. [Google Scholar] [CrossRef]
- Hansson, C. Allergic contact dermatitis from N-(1,3-dimethylbutyl)-N’-phenyl-p-phenylenediamine and from compounds in polymerized 2,2,4-trimethyl-1,2-dihydroquinoline. Contact Dermat. 1994, 30, 114–115. [Google Scholar] [CrossRef]
- Herve-Bazin, B.; Gradiski, D.; Duprat, P.; Marionac, B.; Foussereau, J.; Cavelier, C.; Btebr, P. Occupational eczema from N-isopropyl-N’-phenylparaphenylenediamine (IPPD) and N-dimethyl-1,3 butyl-N’-phenylparaphenylenediamine (DMPPD) in tyres. Contact Dermat. 1977, 3, 1–15. [Google Scholar] [CrossRef]
- Takagi, A.; Takada, K.; Sai, K.; Ochiai, T.; Matsumoto, K.; Sekita, K.; Momma, J.; Aida, Y.; Saitoh, M.; Naitoh, K.; et al. Acute, subchronic and chronic toxicity studies of a synthetic antioxidant, 2, 2′-methylenebis(4-meyhyl-6-tert-butylphenol) in rats. J. Toxicol. Sci. 1994, 19, 77–89. [Google Scholar] [CrossRef] [Green Version]
- Kawasaki, Y.; Umemura, T.; Saito, M.; Momma, J.; Matsushima, Y.; Sekiguchi, H.; Matsumoto, M.; Sakemi, K.; Isama, K.; Inoue, T.; et al. Toxicity study of a rubber antioxidant, 2-mercaptobenzimidazole, by repeated oral administration to rats. J. Toxicol. Sci. 1998, 23, 53–68. [Google Scholar] [CrossRef]
- Sakemi, K.; Usami, M.; Mitsunaga, K.; Ohno, Y.; Tsuda, M. Comparative toxicokinetic study of rubber antioxidants, 2-mercaptobenzimidazole and 2-mercaptomethylbenzimidazole, by single oral administration in rats. J. Toxicol. Sci. 1999, 24, 399–405. [Google Scholar] [CrossRef]
- Riquet, A.M.; Breysse, C.; Dahbi, L.; Loriot, C.; Severin, I.; Chagnon, M.C. The consequences of physical post-treatments (microwave and electron-beam) on food/packaging interactions: A physicochemical and toxicological approach. Food Chem. 2016, 199, 59–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, R.R.; Linville, T.W.; Whynot, A.D.; Brazel, C.S. Evaluating the toxicity of bDtBPP on CHO-K1 cells for testing of single-use bioprocessing systems considering media selection, cell culture volume, mixing, and exposure duration. Biotechnol. Prog. 2016, 32, 1318–1323. [Google Scholar] [CrossRef]
- Peng, W.J.; Liu, C.S.; Chen, D.Q.; Duan, X.B.; Zhong, L.Q. Exposure to N-(1,3-dimethylbutyl)-N’-phenyl-p-phenylenediamine (6PPD) affects the growth and development of zebrafish embryos/larvae. Ecotoxicol. Environ. Saf. 2022, 232, 113221. [Google Scholar] [CrossRef] [PubMed]
- Hiki, K.; Asahina, K.; Kato, K.; Yamagishi, T.; Omagari, R.; Iwasaki, Y.; Watanabe, H.; Yamamoto, H. Acute toxicity of a tire rubber-derived chemical, 6PPD quinone, to freshwater fish and crustacean species. Environ. Sci. Technol. Lett. 2021, 8, 779–784. [Google Scholar] [CrossRef]
- Varshney, S.; Gora, A.H.; Siriyappagouder, P.; Kiron, V.; Olsvik, P.A. Toxicological effects of 6PPD and 6PPD quinone in zebrafish larvae. J. Hazard. Mater. 2022, 424, 127623. [Google Scholar] [CrossRef] [PubMed]
- Klauschies, T.; Isanta-Navarro, J. The joint effects of salt and 6PPD contamination on a freshwater herbivore. Sci. Total Environ. 2022, 829, 154675. [Google Scholar] [CrossRef]
- Zhong, L.; Peng, W.; Liu, C.; Gao, L.; Chen, D.; Duan, X. IPPD-induced growth inhibition and its mechanism in zebrafish. Ecotoxicol. Environ. Saf. 2022, 239, 113614. [Google Scholar] [CrossRef]
- Brinkmann, M.; Montgomery, D.; Selinger, S.; Miller, J.G.P.; Stock, E.; Alcaraz, A.J.; Challis, J.K.; Weber, L.; Janz, D.; Hecker, M.; et al. Acute toxicity of the tire rubber-derived chemical 6PPD-quinone to four fishes of commercial, cultural, and ecological importance. Environ. Sci. Technol. Lett. 2022, 9, 333–338. [Google Scholar] [CrossRef]
- Mahoney, H.; da Silva Junior, F.C.; Roberts, C.; Schultz, M.; Ji, X.; Alcaraz, A.J.; Montgomery, D.; Selinger, S.; Challis, J.K.; Giesy, J.P.; et al. Exposure to the tire rubber-derived contaminant 6PPD-Quinone causes mitochondrial dysfunction in vitro. Environ. Sci. Technol. Lett. 2022, 9, 765–771. [Google Scholar] [CrossRef]
- Tian, Z.; Gonzalez, M.; Rideout, C.A.; Zhao, H.N.; Hu, X.; Wetzel, J.; Mudrock, E.; James, C.A.; McIntyre, J.K.; Kolodziej, E.P. 6PPD-quinone: Revised toxicity assessment and quantification with a commercial standard. Environ. Sci. Technol. Lett. 2022, 9, 140–146. [Google Scholar] [CrossRef]
- Gillis, P.L.; Parrott, J.L.; Helm, P. Environmental fate and effects of road run-off. Arch. Environ. Contam. Toxicol. 2022, 82, 159–161. [Google Scholar] [CrossRef] [PubMed]
- Rauert, C.; Vardy, S.; Daniell, B.; Charlton, N.; Thomas, K.V. Tyre additive chemicals, tyre road wear particles and high production polymers in surface water at 5 urban centres in Queensland, Australia. Sci. Total Environ. 2022, 852, 158468. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, M.; Umemura, T.; Kawasaki, Y.; Momma, J.; Matsushima, Y.; Sakemi, K.; Isama, K.; Kitajima, S.; Ogawa, Y.; Hasegawa, R.; et al. Toxicity study of a rubber antioxidant, mixture of 2-mercaptomethylbenzimidazoles, by repeated oral administration to rats. Food Chem. Toxicol. 1999, 37, 777–787. [Google Scholar] [CrossRef]
- Huntink, N.M.; Datta, R.N.; Noordermeer, J.W.M. Addressing durability of rubber compounds. Rubber Chem. Technol. 2004, 77, 476–511. [Google Scholar] [CrossRef]
- Rødland, E.S.; Lind, O.C.; Reid, M.J.; Heier, L.S.; Okoffo, E.D.; Rauert, C.; Thomas, K.V.; Meland, S. Occurrence of tire and road wear particles in urban and peri-urban snowbanks, and their potential environmental implications. Sci. Total Environ. 2022, 824, 153785. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Liu, L.H.; Xu, D.Y.; Zhang, B.H.; Li, J.J.; Gao, B. Small-sized microplastics (<500 μm) in roadside soils of Beijing, China: Accumulation, stability, and human exposure risk. Environ. Pollut. 2022, 304, 119121. [Google Scholar] [CrossRef]
- Popick, H.; Brinkmann, M.; McPhedran, K. Assessment of stormwater discharge contamination and toxicity for a cold-climate urban landscape. Environ. Sci. Eur. 2022, 34, 43. [Google Scholar] [CrossRef]
- Jan, K.P.; LöHr, A.J.; Frank, V.B.; Ad, R. Wear and tear of tyres: A stealthy source of microplastics in the environment. Int. J. Environ. Res. Public Health 2017, 14, 1265. [Google Scholar]
- Roy, P.; Mohanty, A.K.; Misra, M. Microplastics in ecosystems: Their implications and mitigation pathways. Environ. Sci. Adv. 2022, 1, 9–29. [Google Scholar] [CrossRef]
- Rauert, C.; Charlton, N.; Okoffo, E.D.; Stanton, R.S.; Agua, A.R.; Pirrung, M.C.; Thomas, K.V. Concentrations of tire additive chemicals and tire road wear particles in an Australian urban tributary. Environ. Sci. Technol. 2022, 56, 2421–2431. [Google Scholar] [CrossRef] [PubMed]
- Johannessen, C.; Helm, P.; Metcalfe, C.D. Runoff of the tire-wear compound, hexamethoxymethyl-melamine into urban watersheds. Arch. Environ. Contam. Toxicol. 2022, 82, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Johannessen, C.; Metcalfe, C.D. The occurrence of tire wear compounds and their transformation products in municipal wastewater and drinking water treatment plants. Environ. Monit. Assess. 2022, 194, 731. [Google Scholar] [CrossRef] [PubMed]
- Nedrich, S. Preliminary investigation of 6PPD-quinone in surface water and standing road water in Michigan. In Proceedings of the 2022 Emerging Contaminants in the Environment Conference (ECEC22), Stockholm, Sweden, 27–28 April 2022. [Google Scholar]
- Zhang, R.; Zhao, S.; Liu, X.; Tian, L.; Mo, Y.; Yi, X.; Liu, S.; Liu, J.; Li, J.; Zhang, G. Aquatic Environmental Fates and Risks of Benzotriazoles, Benzothiazoles, and P-Phenylenediamines in a Catchment Providing Water to a Megacity of China. Available online: https://ssrn.com/abstract=4164982 (accessed on 23 September 2022).
- Johannessen, C.; Helm, P.; Metcalfe, C.D. Detection of selected tire wear compounds in urban receiving waters. Environ. Pollut. 2021, 287, 117659. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, C.; Zhang, W.; Qi, Z.; Song, Y.; Zhu, L.; Dong, C.; Chen, J.; Cai, Z. p-Phenylenediamine Antioxidants in PM2.5: The Underestimated Urban Air Pollutants. Environ. Sci. Technol. 2022, 56, 6914–6921. [Google Scholar] [CrossRef]
- Cao, G.; Wang, W.; Zhang, J.; Wu, P.; Zhao, X.; Yang, Z.; Hu, D.; Cai, Z. New evidence of rubber-derived quinones in water, air, and soil. Environ. Sci. Technol. 2022, 56, 4142–4150. [Google Scholar] [CrossRef]
- Cao, G.; Zhang, J.; Wang, W.; Wu, P.; Ru, Y.; Cai, Z. Mass spectrometry analysis of a ubiquitous tire rubber-derived quinone in the environment. TrAC Trends Anal. Chem. 2022, 157, 116756. [Google Scholar] [CrossRef]
- Huang, W.; Shi, Y.M.; Huang, J.L.; Deng, C.L.; Tang, S.Q.; Liu, X.T.; Chen, D. Occurrence of substituted p-Phenylenediamine antioxidants in dusts. Environ. Sci. Technol. Lett. 2021, 8, 381–385. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Xu, T.T.; Ye, D.M.; Lin, Z.Z.; Wang, F.; Guo, Y. Widespread N-(1,3-Dimethylbutyl)-N’-phenyl-p-phenylenediamine quinone in size-fractioned atmospheric particles and dust of different indoor environments. Environ. Sci. Technol. Lett. 2022, 9, 420–425. [Google Scholar] [CrossRef]
- Habibi, N.; Uddin, S.; Fowler, S.W.; Behbehani, M. Microplastics in the atmosphere: A review. J. Environ. Expo. Assess. 2022, 1, 6. [Google Scholar] [CrossRef]
- Liu, R.P.; Li, Z.Z.; Liu, F.; Dong, Y.; Jiao, J.G.; Sun, P.P.; El-Wardany, R.M. Microplastic pollution in Yellow River, China: Current status and research progress of biotoxicological effects. Geol. China 2021, 4, 585–592. [Google Scholar]
- Klöckner, P.; Seiwert, B.; Wagner, S.; Reemtsma, T. Organic markers of tire and road wear particles in sediments and soils: Transformation products of major antiozonants as promising candidates. Environ. Sci. Technol. 2021, 55, 11723–11732. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.X.; Zhou, X.Y.; Su, Y.; Wang, H.M.; Yu, R.L.; Zhou, S.F.; Xu, E.G.; Xing, B.S. Environmental occurrence, fate, impact, and potential solution of tire microplastics: Similarities and differences with tire wear particles. Sci. Total Environ. 2021, 795, 148902. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, J.K.; Prat, J.; Cameron, J.; Wetzel, J.; Mudrock, E.; Peter, K.T.; Tian, Z.Y.; Mackenzie, C.; Lundin, J.; Stark, J.D.; et al. Treading water: Tire wear particle leachate recreates an urban runoff mortality syndrome in coho but not chum salmon. Environ. Sci. Technol. 2021, 55, 11767–11774. [Google Scholar] [CrossRef] [PubMed]
- Parker, B.W.; Beckingham, B.A.; Ingram, B.C.; Ballenger, J.C.; Weinstein, J.E.; Sancho, G. Microplastic and tire wear particle occurrence in fishes from an urban estuary: Influence of feeding characteristics on exposure risk. Mar. Pollut. Bull. 2020, 160, 111539. [Google Scholar] [CrossRef]
- Revel, M.; Chatel, A.; Mouneyrac, C. Micro(nano)plastics: A threat to human health? Curr. Opin. Environ. Sci. Health 2018, 1, 17–23. [Google Scholar] [CrossRef]
- Peter, K.T.; Tian, Z.; Wu, C.; Lin, P.; White, S.; Du, B.; McIntyre, J.K.; Scholz, N.L.; Kolodziej, E.P. Using high-resolution mass spectrometry to identify organic contaminants linked to urban stormwater mortality syndrome in coho salmon. Environ. Sci. Technol. 2018, 52, 10317–10327. [Google Scholar] [CrossRef]
- Johannessen, C.; Helm, P.; Lashuk, B.; Yargeau, V.; Metcalfe, C.D. The tire wear compounds 6PPD-quinone and 1,3-diphenylguanidine in an urban watershed. Arch. Environ. Contam. Toxicol. 2022, 82, 171–179. [Google Scholar] [CrossRef]
- Dsikowitzky, L.; Schwarzbauer, J. Hexa(methoxymethyl)melamine: An emerging contaminant in German rivers. Water Environ. Res. 2015, 87, 461–469. [Google Scholar] [CrossRef]
- Liu, R.; Li, Y.; Lin, Y.; Ruan, T.; Jiang, G. Emerging aromatic secondary amine contaminants and related derivatives in various dust matrices in China. Ecotoxicol. Environ. Saf. 2019, 170, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Li, J.; Du, B.; Pan, Z.; Liu, L.-Y.; Zeng, L. E-Waste recycling emits large quantities of emerging aromatic amines and organophosphites: A poorly recognized source for another two classes of synthetic antioxidants. Environ. Sci. Technol. Lett. 2022, 9, 625–631. [Google Scholar] [CrossRef]
- Ng, B.; Quinete, N.; Gardinali, P. Differential Organic Contaminant Ionization Source Detection and Identification in Environmental Waters by Non-Targeted Analysis. Environ. Toxicol. Chem. 2022, 41, 1154–1164. [Google Scholar] [CrossRef] [PubMed]
- Johannessen, C.; Parnis, J.M. Environmental modelling of hexamethoxymethylmelamine, its transformation products, and precursor compounds: An emerging family of contaminants from tire wear. Chemosphere 2021, 280, 130914. [Google Scholar] [CrossRef] [PubMed]
- Marchant, C.A. Computational toxicology: A tool for all industries. WIREs Comput. Mol. Sci. 2012, 2, 424–434. [Google Scholar] [CrossRef]
- Merlot, C. Computational toxicology—A tool for early safety evaluation. Drug Discov. Today 2010, 15, 16–22. [Google Scholar] [CrossRef]
- Nigsch, F.; Macaluso, N.J.M.; Mitchell, J.B.O.; Zmuidinavicius, D. Computational toxicology: An overview of the sources of data and of modelling methods. Expert Opin. Drug Metab. Toxicol. 2009, 5, 1–14. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Hao, Y.; Yang, Z.; Li, W.; Xie, W.; Huang, Y.; Wang, D.; He, Y.; Liang, Y.; Matsiko, J.; et al. Rubber Antioxidants and Their Transformation Products: Environmental Occurrence and Potential Impact. Int. J. Environ. Res. Public Health 2022, 19, 14595. https://doi.org/10.3390/ijerph192114595
Xu J, Hao Y, Yang Z, Li W, Xie W, Huang Y, Wang D, He Y, Liang Y, Matsiko J, et al. Rubber Antioxidants and Their Transformation Products: Environmental Occurrence and Potential Impact. International Journal of Environmental Research and Public Health. 2022; 19(21):14595. https://doi.org/10.3390/ijerph192114595
Chicago/Turabian StyleXu, Jing, Yanfen Hao, Zhiruo Yang, Wenjuan Li, Wenjing Xie, Yani Huang, Deliang Wang, Yuqing He, Yong Liang, Julius Matsiko, and et al. 2022. "Rubber Antioxidants and Their Transformation Products: Environmental Occurrence and Potential Impact" International Journal of Environmental Research and Public Health 19, no. 21: 14595. https://doi.org/10.3390/ijerph192114595
APA StyleXu, J., Hao, Y., Yang, Z., Li, W., Xie, W., Huang, Y., Wang, D., He, Y., Liang, Y., Matsiko, J., & Wang, P. (2022). Rubber Antioxidants and Their Transformation Products: Environmental Occurrence and Potential Impact. International Journal of Environmental Research and Public Health, 19(21), 14595. https://doi.org/10.3390/ijerph192114595