Forewarned Is Forearmed: Machine Learning Algorithms for the Prediction of Catheter-Induced Coronary and Aortic Injuries
Abstract
:1. Introduction
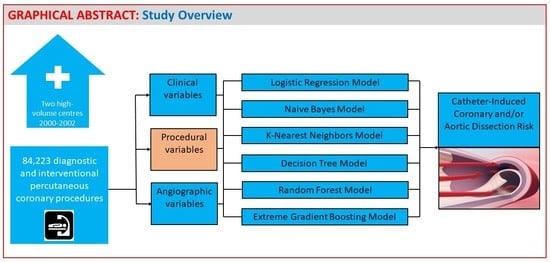
2. Materials and Methods
2.1. Study Population and Data Collection
2.2. Definitions
2.3. Statistical Analysis
3. Results
3.1. Dissection Incidence
3.2. Clinical and Procedural Characteristics of the Dissection Cohort
3.3. Logistic Regression Modelling
3.4. Multivariable Logistic Regression vs. Other Machine Learning Methods
3.5. Model Performance Comparison
4. Discussion
4.1. Traditional Risk Prediction Modelling
4.2. Discriminator: Risk by the Machines
4.3. Anatomical Determinants of Dissection
4.4. Procedural Risk Factors
4.5. Management
5. Study Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramasamy, A.; Bajaj, R.; Jones, D.A.; Amersey, R.; Mathur, A.; Baumbach, A.; Bourantas, C.V.; O’Mahony, C. Iatrogenic Catheter-Induced Ostial Coronary Artery Dissections: Prevalence, Management, and Mortality from a Cohort of 55,968 Patients over 10 Years. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2021, 98, 649–655. [Google Scholar] [CrossRef]
- Amano, H.; Kubo, S.; Osakada, K.; Miura, K.; Ohya, M.; Shimada, T.; Murai, R.; Tada, T.; Tanaka, H.; Fuku, Y.; et al. Clinical Outcomes and Angiographic Results of Bailout Stenting for Guide Catheter-Induced Iatrogenic Coronary Artery Dissection—Impact of Stent Type. Circ. J. Off. J. Jpn. Circ. Soc. 2020, 84, 1746–1753. [Google Scholar] [CrossRef]
- Dunning, D.W.; Kahn, J.K.; Hawkins, E.T.; O’Neill, W.W. Iatrogenic Coronary Artery Dissections Extending into and Involving the Aortic Root. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2000, 51, 387–393. [Google Scholar] [CrossRef]
- Hiraide, T.; Sawano, M.; Shiraishi, Y.; Ueda, I.; Numasawa, Y.; Noma, S.; Negishi, K.; Ohki, T.; Yuasa, S.; Hayashida, K.; et al. Impact of Catheter-Induced Iatrogenic Coronary Artery Dissection with or without Postprocedural Flow Impairment: A Report from a Japanese Multicenter Percutaneous Coronary Intervention Registry. PLoS ONE 2018, 13, e0204333. [Google Scholar] [CrossRef]
- Boyle, A.J.; Chan, M.; Dib, J.; Resar, J. Catheter-Induced Coronary Artery Dissection: Risk Factors, Prevention and Management. J. Invasive Cardiol. 2006, 18, 500–503. [Google Scholar]
- Andreou, A.Y.; Avraamides, P.C.; Andoniade, T.; Theodorou, S.; Mavroudis, C.; Kyriakou, T. Iatrogenic Left Main Coronary Artery Dissection: Mind the Catheter Tip. Cardiovasc. Med. 2016, 19, 264–271. [Google Scholar] [CrossRef] [Green Version]
- Peterson, E.D.; Dai, D.; DeLong, E.R.; Brennan, J.M.; Singh, M.; Rao, S.V.; Shaw, R.E.; Roe, M.T.; Ho, K.K.L.; Klein, L.W.; et al. NCDR Registry Participants. Contemporary Mortality Risk Prediction for Percutaneous Coronary Intervention: Results from 588,398 Procedures in the National Cardiovascular Data Registry. J. Am. Coll. Cardiol. 2010, 55, 1923–1932. [Google Scholar] [CrossRef] [Green Version]
- Niimi, N.; Shiraishi, Y.; Sawano, M.; Ikemura, N.; Inohara, T.; Ueda, I.; Fukuda, K.; Kohsaka, S. Machine Learning Models for Prediction of Adverse Events after Percutaneous Coronary Intervention. Sci. Rep. 2022, 12, 6262. [Google Scholar] [CrossRef]
- Al’Aref, S.J.; Singh, G.; van Rosendael, A.R.; Kolli, K.K.; Ma, X.; Maliakal, G.; Pandey, M.; Lee, B.C.; Wang, J.; Xu, Z.; et al. Determinants of In-Hospital Mortality After Percutaneous Coronary Intervention: A Machine Learning Approach. J. Am. Heart Assoc. 2019, 8, e011160. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.-C.; Chen, K.-Y.; Li, S.-J.; Liu, C.-K.; Lin, Y.-C.; Chen, M. Implementing an Ensemble Learning Model with Feature Selection to Predict Mortality among Patients Who Underwent Three-Vessel Percutaneous Coronary Intervention. Appl. Sci. 2022, 12, 8135. [Google Scholar] [CrossRef]
- Hsieh, M.-H.; Lin, S.-Y.; Lin, C.-L.; Hsieh, M.-J.; Hsu, W.-H.; Ju, S.-W.; Lin, C.-C.; Hsu, C.Y.; Kao, C.-H. A Fitting Machine Learning Prediction Model for Short-Term Mortality Following Percutaneous Catheterization Intervention: A Nationwide Population-Based Study. Ann. Transl. Med. 2019, 7, 732. [Google Scholar] [CrossRef]
- Brennan, J.M.; Curtis, J.P.; Dai, D.; Fitzgerald, S.; Khandelwal, A.K.; Spertus, J.A.; Rao, S.V.; Singh, M.; Shaw, R.E.; Ho, K.K.L.; et al. National Cardiovascular Data Registry. Enhanced Mortality Risk Prediction with a Focus on High-Risk Percutaneous Coronary Intervention: Results from 1,208,137 Procedures in the NCDR (National Cardiovascular Data Registry). JACC Cardiovasc. Interv. 2013, 6, 790–799. [Google Scholar] [CrossRef] [Green Version]
- Castro-Dominguez, Y.S.; Wang, Y.; Minges, K.E.; McNamara, R.L.; Spertus, J.A.; Dehmer, G.J.; Messenger, J.C.; Lavin, K.; Anderson, C.; Blankinship, K.; et al. Predicting In-Hospital Mortality in Patients Undergoing Percutaneous Coronary Intervention. J. Am. Coll. Cardiol. 2021, 78, 216–229. [Google Scholar] [CrossRef]
- Inohara, T.; Kohsaka, S.; Yamaji, K.; Ishii, H.; Amano, T.; Uemura, S.; Kadota, K.; Kumamaru, H.; Miyata, H.; Nakamura, M. Risk Stratification Model for In-Hospital Death in Patients Undergoing Percutaneous Coronary Intervention: A Nationwide Retrospective Cohort Study in Japan. BMJ Open 2019, 9, e026683. [Google Scholar] [CrossRef] [Green Version]
- Wall, J.J.S.; Iqbal, J.; Andrews, M.; Teare, D.; Ghobrial, M.; Hinton, T.; Turton, S.; Quffa, L.; El-Omar, M.; Fraser, D.G.; et al. Development and Validation of a Clinical Risk Score to Predict Mortality after Percutaneous Coronary Intervention. Open Heart 2017, 4, e000576. [Google Scholar] [CrossRef]
- Moore, A.; Bell, M. XGBoost, A Novel Explainable AI Technique, in the Prediction of Myocardial Infarction: A UK Biobank Cohort Study. Clin. Med. Insights Cardiol. 2022, 16, 11795468221133612. [Google Scholar] [CrossRef]
- Shorrock, D.; Michael, T.T.; Patel, V.; Kotsia, A.; Rangan, B.V.; Abdullah, S.A.; Grodin, J.M.; Banerjee, A.; Brilakis, E.S. Frequency and Outcomes of Aortocoronary Dissection during Percutaneous Coronary Intervention of Chronic Total Occlusions: A Case Series and Systematic Review of the Literature. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2014, 84, 670–675. [Google Scholar] [CrossRef] [Green Version]
- Rathore, S.; Katoh, O.; Matsuo, H.; Terashima, M.; Tanaka, N.; Kinoshita, Y.; Kimura, M.; Tsuchikane, E.; Nasu, K.; Ehara, M.; et al. Retrograde Percutaneous Recanalization of Chronic Total Occlusion of the Coronary Arteries: Procedural Outcomes and Predictors of Success in Contemporary Practice. Circ. Cardiovasc. Interv. 2009, 2, 124–132. [Google Scholar] [CrossRef] [Green Version]
- Rathore, S.; Matsuo, H.; Terashima, M.; Kinoshita, Y.; Kimura, M.; Tsuchikane, E.; Nasu, K.; Ehara, M.; Asakura, Y.; Katoh, O.; et al. Procedural and In-Hospital Outcomes after Percutaneous Coronary Intervention for Chronic Total Occlusions of Coronary Arteries 2002 to 2008: Impact of Novel Guidewire Techniques. JACC Cardiovasc. Interv. 2009, 2, 489–497. [Google Scholar] [CrossRef]
- Wu, C.-J.; Fang, H.-Y.; Cheng, C.-I.; Hussein, H.; Abdou, S.M.; Youssef, A.A.; Bhasin, A.; Yang, C.-H.; Chen, C.-J.; Hsieh, Y.-K.; et al. The Safety and Feasibility of Bilateral Radial Approach in Chronic Total Occlusion Percutaneous Coronary Intervention. Int. Heart J. 2011, 52, 131–138. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Moreno, S.; Sabaté, M.; Jiménez-Quevedo, P.; Vázquez, P.; Alfonso, F.; Angiolillo, D.J.; Hernández-Antolín, R.; Moreno, R.; Bañuelos, C.; Escaned, J.; et al. Iatrogenic Dissection of the Ascending Aorta Following Heart Catheterisation: Incidence, Management and Outcome. EuroIntervention J. Eur. Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2006, 2, 197–202. [Google Scholar]
- Eshtehardi, P.; Adorjan, P.; Togni, M.; Tevaearai, H.; Vogel, R.; Seiler, C.; Meier, B.; Windecker, S.; Carrel, T.; Wenaweser, P.; et al. Iatrogenic Left Main Coronary Artery Dissection: Incidence, Classification, Management, and Long-Term Follow-Up. Am. Heart J. 2010, 159, 1147–1153. [Google Scholar] [CrossRef]
- Faggian, G.; Santini, F.; Petrilli, G.; Mazzucco, A.; Rigatelli, G.; Cardaioli, P.; Roncon, L. Left Main Coronary Stenosis as a Late Complication of Percutaneous Angioplasty:An Old Problem, but Still a Problem. J. Geriatr. Cardiol. 2009, 6, 26–30. [Google Scholar]
- López-Mínguez, J.R.; Climent, V.; Yen-Ho, S.; González-Fernández, R.; Nogales-Asensio, J.M.; Sánchez-Quintana, D. Structural features of the sinus of valsalva and the proximal portion of the coronary arteries: Their relevance to retrograde aortocoronary dissection. Rev. Esp. Cardiol. 2006, 59, 696–702. [Google Scholar] [CrossRef]
- Harding, S.A.; Fairley, S.L. Catheter-Induced Coronary Dissection: Keep Calm and Don’t Inject. JACC Case Rep. 2019, 1, 113–115. [Google Scholar] [CrossRef]
- Alsanjari, O.; Myat, A.; Cockburn, J.; Karamasis, G.V.; Hildick-Smith, D.; Kalogeropoulos, A.S. A Case of an Obstructive Intramural Haematoma during Percutaneous Coronary Intervention Successfully Treated with Intima Microfenestrations Utilising a Cutting Balloon Inflation Technique. Case Rep. Cardiol. 2018, 2018, 4875041. [Google Scholar] [CrossRef]
- Costello-Boerrigter, L.C.; Salomon, C.; Bufe, A.; Lapp, H. The novel use of retrograde CTO PCI techniques as a rescue strategy for an acute right coronary artery occlusion due to iatrogenic dissection. J Cardiol. Cases. 2017, 17, 89–91. [Google Scholar] [CrossRef] [Green Version]
- Hashmani, S.; Tuzcu, E.; Hasan, F. Successful Bail-Out Stenting for Iatrogenic Right Coronary Artery Dissection in a Young Male. JACC Case Rep. 2019, 1, 108–112. [Google Scholar] [CrossRef]
- Klaudel, J.; Glaza, M.; Klaudel, B.; Trenkner, W.; Pawłowski, K.; Szołkiewicz, M. Catheter-induced coronary artery and aortic dissections. A study of the mechanisms, risk factors, and propagation causes. Cardiol J. 2022. [Google Scholar] [CrossRef]



| Dissected Vessel; n = 117 | |||
|---|---|---|---|
| LCA | 58 | 49.6 | |
| RCA | 57 | 48.7 | |
| SVG | 2 | 1.7 | |
| Coronary Artery (+SVG) Dissection by NHLBI Type; n = 117 | |||
| Localised: | A-B | 53 | 45.3 |
| Extensive: | C-F | 53 | 45.3 |
| C | 27 | 23.1 | |
| D | 21 | 17.9 | |
| E | 5 | 4.3 | |
| F | 11 | 9.4 | |
| Aortic Involvement; n = 19 | |||
| Isolated sinus of Valsalva dissection | 7 | 36.8 | |
| Aortocoronary dissection | 12 | 63.2 | |
| Aortic dissection starting in RCA and/or involving right SoV | 13 | 68.4 | |
| Aortic dissection starting in LCA and/or involving left SoV | 6 | 31.6 | |
| Aortic Dissection by Dunning Classification; n = 19 | |||
| Type I | 10 | 52.6 | |
| Type II | 5 | 26.3 | |
| Type III | 4 | 21.1 | |
| Treatment; n = 124 | |||
| Conservative | 39 | 31.5 | |
| Intervention | 83 | 66.9 | |
| Surgery | 2 | 1.6 | |
| n = 124 | ||
|---|---|---|
| Age | 69.1 | 11.9 |
| Female gender | 59 | 47.6 |
| Body mass index | 27.4 | 4.5 |
| Left ventricular ejection fraction | 48.9 | 11.8 |
| Comorbidities and Medical History | ||
| Diabetes mellitus | 27 | 21.8 |
| Hypertension | 75 | 60.5 |
| Chronic renal failure | 14 | 11.3 |
| Peripheral arterial disease | 9 | 7.3 |
| Stroke | 9 | 7.3 |
| Chronic obstructive pulmonary disease | 7 | 5.6 |
| Prior myocardial infarction | 28 | 22.6 |
| Prior PCI | 37 | 29.8 |
| Prior CABG | 6 | 4.8 |
| Initial Presentation and in-Hospital Mortality | ||
| Non-ACS | 33 | 26.6 |
| NSTE-ACS | 40 | 32.3 |
| STE-ACS | 51 | 41.1 |
| Prehospital cardiac arrest | 5 | 4 |
| Shock at admission | 6 | 4.8 |
| In-hospital death | 7 | 5.6 |
| In-hospital death due to dissection | 3 | 2.4 |
| Procedural characteristics | ||
| Femoral access | 25 | 20.2 |
| Radial access (incl. ulnar and brachial access) | 99 | 79.8 |
| Coronary angiography | 20 | 16.1 |
| PCI | 104 | 83.9 |
| CTO angioplasty | 9 | 7.3 |
| Algorithm | Accuracy | Precision | Recall | f1-Score | |
|---|---|---|---|---|---|
| 1 | logistic_regression | 0.956286 | 0.043805 | 0.539876 | 0.079809 |
| 2 | decision_tree | 0.997499 | 0.690994 | 0.330623 | 0.445231 |
| 3 | random_forest | 0.997524 | 0.725000 | 0.322687 | 0.443662 |
| 4 | naive_bayes | 0.958517 | 0.050157 | 0.451607 | 0.085814 |
| 5 | knn | 0.996715 | 0.429293 | 0.168990 | 0.235908 |
| 6 | xgboost | 0.997695 | 0.747911 | 0.362563 | 0.488273 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klaudel, J.; Klaudel, B.; Glaza, M.; Trenkner, W.; Derejko, P.; Szołkiewicz, M. Forewarned Is Forearmed: Machine Learning Algorithms for the Prediction of Catheter-Induced Coronary and Aortic Injuries. Int. J. Environ. Res. Public Health 2022, 19, 17002. https://doi.org/10.3390/ijerph192417002
Klaudel J, Klaudel B, Glaza M, Trenkner W, Derejko P, Szołkiewicz M. Forewarned Is Forearmed: Machine Learning Algorithms for the Prediction of Catheter-Induced Coronary and Aortic Injuries. International Journal of Environmental Research and Public Health. 2022; 19(24):17002. https://doi.org/10.3390/ijerph192417002
Chicago/Turabian StyleKlaudel, Jacek, Barbara Klaudel, Michał Glaza, Wojciech Trenkner, Paweł Derejko, and Marek Szołkiewicz. 2022. "Forewarned Is Forearmed: Machine Learning Algorithms for the Prediction of Catheter-Induced Coronary and Aortic Injuries" International Journal of Environmental Research and Public Health 19, no. 24: 17002. https://doi.org/10.3390/ijerph192417002
APA StyleKlaudel, J., Klaudel, B., Glaza, M., Trenkner, W., Derejko, P., & Szołkiewicz, M. (2022). Forewarned Is Forearmed: Machine Learning Algorithms for the Prediction of Catheter-Induced Coronary and Aortic Injuries. International Journal of Environmental Research and Public Health, 19(24), 17002. https://doi.org/10.3390/ijerph192417002