Plant Adaptability and Vegetation Differentiation in the Coastal Beaches of Yellow–Bohai Sea in China
Abstract
:1. Introduction
- The roles of the main soil factors in vegetation differentiation, and their regional and seasonal differences; and
- Key species’ strategic features and the mechanism of vegetation differentiation.
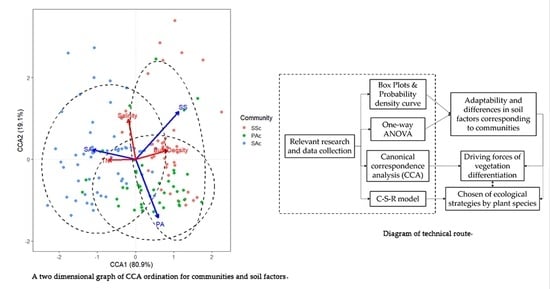
2. Materials and Methods
2.1. Study Area
2.2. Statistical Analysis
- The study area is limited to the tidal flat of the YBS in China from 30°–42° N;
- The exact location of the study site and the time of sampling (at least specific to season) should be specified in the paper; and
- At least one type of data of soil physical and chemical properties should be included, such as soil bulk density (BD), soil salinity, total organic carbon (TOC), total nitrogen (TN), total phosphorus (TP), pH, and so forth, which correspond to the three typical mono-dominant communities.
3. Results
3.1. Characteristics and Variation of Soil Factors in Typical Communities
3.2. Redundancy Analysis between Soil Factors and Communities
3.3. Distribution of Three Communities along Soil Factor Gradient
4. Discussion
4.1. Regional and Seasonal Differences of Soil Factors for Typical Wetland Communities
4.2. Driving Factors of Vegetation Differentiation
4.3. Plants’ Ecological Strategy and the Mechanism of Vegetation Differentiation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Article | Commuities | Location | Season | Environmental Factors | Citation |
|---|---|---|---|---|---|
| Changes in soil microbial biomass and community composition in coastal wetlands affected by restoration projects in a Chinese delta | SS, PA | North | Spring | pH, Salinity | [39] |
| Characterization of the salt marsh soils and visible−near−infrared spectroscopy along a chronosequence of Spartina alterniflora invasion in a coastal wetland of eastern China | SA | South | Autumn | BD, pH, Salnility, TN | [40] |
| Comparison of phosphorus fractions and phosphatase activities in coastal wetland soils along vegetation zones of Yancheng National Nature Reserve, China | SA, SS, PA | South | Summer | pH, Salinity, TN, TP | [41] |
| Consequences of short−term C−4 plant Spartina alterniflora invasions for soil organic carbon dynamics in a coastal wetland of Eastern China | SA, SS, PA | South | Spring | BD, pH, Salnility | [20] |
| Decomposition processes in coastal wetlands: the importance of Suaeda salsa community for soil cellulose decomposition | SS, PA | North | Autumn | Salinity, TN, TP | [42] |
| The effect of biomass variations of Spartina alterniflora on the organic carbon content and composition of a salt marsh in northern Jiangsu Province, China | SA | South | Spring, Summer, Autumn, Winter | TN | [43] |
| Effects of invasion of Spartina alterniflora and exogenous N deposition on N2O emissions in a coastal salt marsh | SA, PA | South | Spring | BD, TN | [19] |
| Effects of Spartina alterniflora invasion and exogenous nitrogen on soil nitrogen mineralization in the coastal salt marshes | SA, SS, PA | South | Summer | BD, Ph, TN | [44] |
| Effects of Spartina alterniflora Invasion on Soil Respiration in the Yangtze River Estuary, China | SA, PA | South | Autumn | pH | [45] |
| Exotic Spartina alterniflora provides compatible habitats for native estuarine crab Sesarma dehaani in the Yangtze River estuary | SA, PA | South | Summer | pH, Salinity | [46] |
| Halophyte Plant Communities Affecting Enzyme Activity and Microbes in Saline Soils of the Yellow River Delta in China | SS, PA | North | Spring, Summer, Autumn | Salinity | [11] |
| The impact of sea embankment reclamation on soil organic carbon and nitrogen pools in invasive Spartina alterniflora and native Suaeda salsa salt marshes in eastern China | SA, SS | South | Autumn | BD, PH, Salinity | [21] |
| Impacts of Age and Expansion Direction of Invasive Spartina alterniflora on Soil Organic Carbon Dynamics in Coastal Salt Marshes Along Eastern China | SA, SS | North | Autumn | BD, pH, Salinity, TN | [18] |
| Impacts of burial by sediment on decomposition and heavy metal concentrations of Suaeda salsa in intertidal zone of the Yellow River estuary, China | SS | North | Spring | pH, Salinity, TN | [47] |
| Impacts of Spartina alterniflora invasion on soil organic carbon and nitrogen pools sizes, stability, and turnover in a coastal salt marsh of eastern China | SA, SS, PA | South | Autumn | BD, PH, Salinity | [48] |
| Plant litter composition selects different soil microbial structures and in turn drives different litter decomposition pattern and soil carbon sequestration capability | SA, PA | South | Autumn | pH, TN | [49] |
| Response of methane emission to invasion of Spartina alterniflora and exogenous N deposition in the coastal salt marsh | SA, SS | South | Spring | BD, TN | [50] |
| Seasonal Dynamics of Trace Elements in Tidal Salt Marsh Soils as Affected by the Flow−Sediment Regulation Regime | SS, PA | North | Spring, Summer, Autumn | BD, Salinity, pH | [12] |
| Short−term C−4 plant Spartina alterniflora invasions change the soil carbon in C−3 plant−dominated tidal wetlands on a growing estuarine Island | SA | South | Autumn | TN | [51] |
| Soil fungal communities vary with invasion by the exotic Spartina alternifolia Loisel. in coastal salt marshes of eastern China | SA, SS, PA | South | Winter | pH, Salinity | [52] |
| Soil organic carbon of degraded wetlands treated with freshwater in the Yellow River Delta, China | SS, PA | North | Spring | PH, Salinity, TN | [24] |
| Two−decade wetland cultivation and its effects on soil properties in salt marshes in the Yellow River Delta, China | SS, PA | North | Winter | BD, Salinity, pH, TN, TP | [53] |
| Analysis on Diversity of Soil Bacterial Community in Original Coastal Wetland of Yancheng, Jiangsu | SA, SS, PA | South | Autumn | pH, TN, TP | [54] |
| The Assessment of Carbon Storage of Vegetation Zones in the Jiuduan Shoal Wetland | SA, PA | South | Spring, Summer, Autumn, Winter | BD | [55] |
| Biologically−Based Availability and Influencing Factors of Soil Phosphorus under Different Vegetation in Coastal Beach Wetlands | SA, PA | South | Spring | pH, TN, TP | [56] |
| Carbon, nitrogen and phosphorus content and ecological stoichiometry of Spartina alterniflora in the tidal flat wetland of Jiaozhou Bay | SA | North | Spring, Summer, Autumn, Winter | BD, Salinity, pH, TN, TP | [57] |
| Characteristics and Factors of Soil Enzyme Activity for Different Plant Communities in Yellow River Delta | SS, PA | North | Summer | Salinity, pH, TN, TP | [58] |
| The characteristics and mechanism of landscape evolution in the coastal wetlands under natural and human influence | SA, SS, PA | South | Spring | Salinity | [59] |
| Characteristics of halophyte and associated soil along aggradational muddycoasts in Jiangsu Province | SA, SS | South | Spring | Salinity, TN, TP | [60] |
| The Characteristics of Surficial Sediments Organic Carbon inYancheng Coastal Wetland | SA, SS, PA | South | Spring | BD, Salinity, pH | [61] |
| Contents of Organic Carbon and Dissolved Organic Carbon and Characteristics of Functional Group Structure in Surface Soils of Salt Marshes in Yellow River Delta | SA, SS, PA | North | Summer | pH, Salinity | [62] |
| The Coupling Relationship between Soil Eco−Processes and Landscape Evolution under the Natural Conditions in Yancheng Coastal Wetland | SA, SS, PA | South | Spring | Salinity | [63] |
| Distribution and Influence factors of soil organic carbon of different land−use types in the Jiangsu coastal areas | SA, SS | South | Autumn | pH, TN, TP | [64] |
| Distribution characteristic and spatial heterogeneity of soil organic carbon on the south coastal of Hangzhou Bay | SA, PA | South | Spring | pH, Salinity, TN | [65] |
| Distribution characteristics of organic carbon and its components in soils under different types of vegetation in wetland of Hangzhou Bay | SA, PA | South | Spring | pH, Salinity | [66] |
| Distribution Characteristics of Phosphorus under Different Vegetation Communities in Salt Marshes of Jiaozhou Bay Communities in Salt Marshes of Jiaozhou Bay | SA, SS, PA | North | Autumn | TP | [67] |
| Diversity survey in rhizosphere of diazotroph in the exotic invasive species Spartina alterniflora and two native species (Phragmites australis and Scripus mariqueter) in the wetlands at Chongming Dongtan in the Yangtze River estuary | SA, PA | South | Spring | pH | [68] |
| Ecological mechanisms of vegetation succession of coastal wetland in Yancheng Nature Reserve | SA, SS, PA | South | Spring | Salinity | [22] |
| Effect of litter decomposition on mineralization of soil organic carbon in the Jiaozhou Bay coastal wetlands | SA, SS, PA | North | Winter | pH, Salinity, TN, TP | [69] |
| Effect of Salt on Soil Nitrogen Mineralization in Coastal Wetland of Liaohe Estuary | SS, PA | North | Spring | pH, TN | [70] |
| Effect of Spartina alterniflora Invasion on Coastal Wetland Soil Carbon Pool and Stability in Subtropical China | SA, PA | South | Summer | BD, pH, Salinity, TN, TP | [71] |
| Effects of plant invasion along a Spartina alterniflora chronosequence on organic carbon dynamics in coastal wetland in north Jiangsu. | SA, SS | South | Autumn | BD | [72] |
| Effects of plant invasion on soil caibon dynamics and CH4 emissions from coastal wetlands | SA, PA | South | Summer | TN | [73] |
| The effects of salt marsh vegetation on soil organic carbon fractions, sources and distribution | SA, SS | South | Summer | BD, pH, Salinity, TN | [74] |
| Effects of Spartina alterniflora Invasion on Soil Carbon Fractions in Mangrove Wetlands of China | SA | South | Summer | BD, pH, Salinity, TN, TP | [75] |
| Effects of Spartina alterniflora invasion in eastern Fujian coastal wetland on the physicochemical properties and enzyme activities of mangrove soil. | SA | South | Autumn | Ph, TN, TP | [76] |
| The Key Factor of Impact on Temporal and Spatial Variation of Soil Organic Matter, TN and TP in Coastal Salt Marsh: Tide and Vegetation | SA, SS | South | Spring, Summer, Autumn | TN, TP | [77] |
| Leaching Characteristics of Soil Dissovled Organic Carbon in Coastal Wetlands of Jiaozhou Bay | SA, SS, PA | North | Summer | BD, pH, Salinity | [78] |
| Morphology and Biomass Distribution of Spartina alterniflora Growing in Different Tidal Flat Habitats in Jiangsu | SA | South | Autumn | pH, Salinity, TN, TP | [79] |
| Nutrient dynamics of litter−soil system during litter decomposition in coastal wetlands of Jiaozhou Bay | SA, SS, PA | North | Autumn | Ph, TN, TP | [80] |
| Relative competitive ability of Spartina alterniflora patches to native species in tidal zone ecotone of north Jiangsu | SA, SS | South | Summer | pH, Salinity | [81] |
| The relative importance and mechanism of soil dissimilatory nitrate reduction to ammonium and denitrification under the change of land use: A case study in chongming dongtan | SA, PA | South | Spring, Summer, Autumn, Winter | BD, pH, Salinity | [82] |
| Research on characteristics of vegetation distribution pattern and soil factors in the intertidal zone of Zhimai River estuary | SA, PA | North | Summer | pH | [83] |
| Respirations and Response in Temperature of Salt Marsh Soil in Different Types of Wetlands Along the Coast of Yancheng | SA, PA, SS | South | Spring | pH, Salinity, TN | [84] |
| The response of organic carbon content to biomass dynamics in Spartina alterniflora marsh. | SA | South | Spring, Summer, Autumn, Winter | TN | [85] |
| Retention Effect of Wetland for Nitrogen and Phosphorus Nutrients in the Coastal Zone of the Yancheng | SA | South | Summer | TN, TP | [86] |
| Soil Quality Evaluation of Bare Flat and Salt Marshes in Jiaozhou Bay Wetlands | SA, SS, PA | North | Summer | BD, pH, Salinity, TN, TP | [87] |
| Spatial Distribution and Influencing Factors of the Biomass of Spartina alterniflora in Coastal Wetlands of Zhejiang | SA | South | Summer | pH, TN, TP | [88] |
| Spatial Heterogeneity of Soil Salinity in Jiangsu Yancheng Wetland National Nature Reserve Rare Birds | SA, SS, PA | South | Spring | Salinity | [89] |
| The stoichiometric characteristics of different plant communities in the Duliujian River estuary | SA, SS, PA | North | Autumn | pH, Salinity | [90] |
| Study on CH4 Emission Fluxes in Hangzhou Bay Coastal Wetland | SA, PA | South | Autumn | PH, Salinity, TN | [91] |
| Study on methane, nitrous oxide and carbon dioxide fluxes and their influencing factors in Hangzhou Bay coastal wetland | SA, PA | South | Autumn | PH, Salinity, TN | [92] |
| Temporaland Spatial Variability of Soil Nutrients in Different Vegetation Zones of Yueqing Bay Coastal Wetlands | SA | South | Summer, Winter | TN, TP | [93] |
| Vertical distribution and seasonal variation of nitrogen, phosphorus elements in Spartina alterniflora wetland of Jiaozhou Bay, Shandong, China | SA | North | Spring, Summer, Autumn | TN, TP | [94] |


| Variables | R2 | Adjusted R2 | F | p Values |
|---|---|---|---|---|
| North | 0.458549 | 0.389132 | ||
| BD | 22.4 | 0.002 ** | ||
| Salinity | 5.7 | 0.032 * | ||
| pH | 1.2 | 0.292 | ||
| TN | 1 | 0.332 | ||
| TP | 0.5 | 0.556 | ||
| South | 0.37053 | 0.3374 | ||
| BD | 19.4 | 0.002 ** | ||
| Salinity | 14.2 | 0.002 ** | ||
| pH | 13.6 | 0.002 ** | ||
| TN | 0.9 | 0.394 | ||
| TP | 0.8 | 0.464 | ||
| Spring | 0.554406 | 0.498707 | ||
| BD | 13.3 | 0.002 ** | ||
| Salinity | 8.8 | 0.002 ** | ||
| pH | 7.4 | 0.004 ** | ||
| TN | 8.7 | 0.002 ** | ||
| TP | 0.1 | 0.86 | ||
| Summer | 0.334422 | 0.253254 | ||
| BD | 15.9 | 0.002 ** | ||
| Salinity | 2.7 | 0.058 † | ||
| pH | 1.7 | unknown | ||
| TN | 0.1 | 0.852 | ||
| TP | 0.1 | 0.852 | ||
| Autumn | 0.504579 | 0.43577 | ||
| BD | 21.8 | 0.002 ** | ||
| Salinity | 10.2 | 0.002 ** | ||
| pH | 0.8 | 0.382 | ||
| TN | 0.5 | 0.594 | ||
| TP | <0.1 | 0.982 | ||
| Winter | 0.731209 | 0.58188 | ||
| BD | 10 | 0.002 ** | ||
| Salinity | 6.2 | 0.014 * | ||
| pH | 2 | 0.184 | ||
| TN | 1.1 | unknown | ||
| TP | 0.5 | 0.576 |
| Statistic | Eigenvalues | Explained Variation (Cumulative) | Pseudo-Canonical Correlation | Explained Fitted Variation (Cumulative) | |
|---|---|---|---|---|---|
| North | Axis 1 | 0.360 | 35.99 | 0.922 | 78.49 |
| Axis 2 | 0.099 | 45.85 | 0.414 | 100 | |
| South | Axis 1 | 0.278 | 27.76 | 0.685 | 74.93 |
| Axis 2 | 0.093 | 37.05 | 0.477 | 100 | |
| Spring | Axis 1 | 0.388 | 38.82 | 0.891 | 70.01 |
| Axis 2 | 0.166 | 55.44 | 0.571 | 100 | |
| Summer | Axis 1 | 0.304 | 30.35 | 0.754 | 90.77 |
| Axis 2 | 0.031 | 33.44 | 0.257 | 100 | |
| Autumn | Axis 1 | 0.477 | 47.72 | 0.908 | 94.58 |
| Axis 2 | 0.027 | 50.46 | 0.255 | 100 | |
| Winter | Axis 1 | 0.499 | 49.87 | 0.906 | 68.2 |
| Axis 2 | 0.233 | 73.12 | 0.769 | 100 | |


References
- Dai, L.J.; Liu, H.Y.; Wang, G.; Wang, C.; Guo, Z.R.; Zhou, Y.; Li, Y.F. Modelling the effects of Spartina alterniflora invasion on the landscape succession of Yancheng coastal natural wetlands, China. PeerJ 2020, 8, e10400. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.J.; Zhang, Z.S.; Xue, Z.S.; Wu, H.T.; Zhang, H.R. Effects of Tidal Channels and Roads on Landscape Dynamic Distribution in the Yellow River Delta, China. Chin. Geogr. Sci. 2020, 30, 170–179. [Google Scholar] [CrossRef] [Green Version]
- An, S.Q.; Gu, B.H.; Zhou, C.F.; Wang, Z.S.; Deng, Z.F.; Zhi, Y.B.; Li, H.L.; Chen, L.; Yu, D.H.; Liu, Y.H. Spartina invasion in China: Implications for invasive species management and future research. Weed Res. 2007, 47, 183–191. [Google Scholar] [CrossRef]
- Edwards, K.R.; Proffitt, C.E. Comparison of wetland structural characteristics between created and natural salt marshes in Southwest Louisiana, USA. Wetlands 2003, 23, 344–356. [Google Scholar] [CrossRef]
- Osgood, D.T.; Santos, M.C.F.V.; Zieman, J. Sediment physico−chemistry associated with natural marsh development on a storm−deposited sand flat. Mar. Ecol. Prog. Ser. 1995, 120, 271–283. [Google Scholar] [CrossRef] [Green Version]
- Barry, A.; Ooi, S.K.; Helton, A.M.; Steven, B.; Elphick, C.S.; Lawrence, B.A. Vegetation Zonation Predicts Soil Carbon Mineralization and Microbial Communities in Southern New England Salt Marshes. Estuaries Coasts 2021, 13, 168–180. [Google Scholar] [CrossRef]
- Hikouei, I.S.; Christian, J.; Kim, S.S.; Sutter, L.A.; Durham, S.A.; Yang, J.D.J.; Vickery, C.G. Use of Random Forest Model to Identify the Relationships among Vegetative Species, Salt Marsh Soil Properties, and Interstitial Water along the Atlantic Coast of Georgia. Infrastructures 2021, 6, 70. [Google Scholar] [CrossRef]
- Castillo, J.M.; Gallego-Tevar, B.; Castellanos, E.M.; Figueroa, M.E.; Davy, A.J. Primary succession in an Atlantic salt marsh: From intertidal flats to mid−marsh platform in 35 years. J. Ecol. 2021, 109, 2909–2921. [Google Scholar] [CrossRef]
- Craft, C. Freshwater Input Structures Soil Properties, Vertical Accretion, and Nutrient Accumulation of Georgia and U.S. Tidal Marshes. Limnol. Oceanogr. 2007, 52, 1220–1230. [Google Scholar] [CrossRef]
- Tang, L.; Li, B.; Zhao, B.; Li, P.; Li, Z.B.; Gao, Y. Invasive Smooth Cordgrass (Spartina alterniflora) Eradication and Native Crab Recovery. Invas. Plant Sci. Mana. 2018, 11, 89–95. [Google Scholar] [CrossRef]
- Cao, D.; Shi, F.; Koike, T.; Lu, Z.; Sun, J. Halophyte Plant Communities Affecting Enzyme Activity and Microbes in Saline Soils of the Yellow River Delta in China. CLEAN—Soil Air Water 2014, 42, 1433–1440. [Google Scholar] [CrossRef]
- Bai, J.; Xiao, R.; Zhao, Q.; Lu, Q.; Wang, J.; Reddy, K.R. Seasonal dynamics of trace elements in tidal salt marsh soils as affected by the flow−sediment regulation regime. PLoS ONE 2017, 9, e107738. [Google Scholar] [CrossRef] [PubMed]
- Studds, C.E.; Kendall, B.E.; Murray, N.J.; Wilson, H.B.; Rogers, D.I.; Clemens, R.S.; Gosbell, K.; Hassell, C.J.; Jessop, R.; Melville, D.S.; et al. Rapid population decline in migratory shorebirds relying on Yellow Sea tidal mudflats as stopover sites. Nat. Commun. 2017, 8, 14895. [Google Scholar] [CrossRef]
- Murray, N.J.; Phinn, S.R.; DeWitt, M.; Ferrari, R.; Johnston, R.; Lyons, M.B.; Clinton, N.; Thau, D.; Fuller, R.A. The global distribution and trajectory of tidal flats. Nature 2019, 565, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.V.; Carrasco, L.R.; Choi, C.Y.; Li, J.; Ma, Z.; Melville, D.S.; Mu, T.; Peng, H.B.; Woodworth, B.K.; Yang, Z.; et al. Multiple habitat use by declining migratory birds necessitates joined−up conservation. Ecol. Evol. 2019, 9, 2505–2515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krebs, C.J. Ecological Methodology; Addison Wesley: California, CA, USA, 1999. [Google Scholar]
- Grime, J.P. The C−S−R model of primary plant strategies—Origins, implications and tests. In Plant Evolutionary Biology; Gottlieb, L.D., Jain, S.K., Eds.; Springer: Dordrecht, The Netherlands, 1988; pp. 371–393. [Google Scholar]
- Qi, X.Z.; Liu, H.Y.; Lin, Z.S.; Liu, X.; Gong, H.B. Impacts of Age and Expansion Direction of Invasive Spartina alterniflora on Soil Organic Carbon Dynamics in Coastal Salt Marshes Along Eastern China. Estuaries Coasts 2019, 42, 1858–1867. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Wang, L.; Xie, X.J.; Huang, L.D.; Wu, Y.H. Effects of invasion of Spartina alterniflora and exogenous N deposition on N2O emissions in a coastal salt marsh. Ecol. Eng. 2013, 58, 77–83. [Google Scholar] [CrossRef]
- Yang, W.; Zhao, H.; Chen, X.L.; Yin, S.L.; Cheng, X.L.; An, S.Q. Consequences of short−term C−4 plant Spartina alterniflora invasions for soil organic carbon dynamics in a coastal wetland of Eastern China. Ecol. Eng. 2013, 61, 50–57. [Google Scholar] [CrossRef]
- Yang, W.; Li, N.; Leng, X.; Qiao, Y.J.; Cheng, X.L.; An, S.Q. The impact of sea embankment reclamation on soil organic carbon and nitrogen pools in invasive Spartina alterniflora and native Suaeda salsa salt marshes in eastern China. Ecol. Eng. 2016, 97, 582–592. [Google Scholar] [CrossRef]
- Yao, C.; Wan, S.; Sun, D.; Qin, P. Ecological mechanisms of vegetation succession of coastal wetland in Yancheng Nature Reserve. Acta Ecol. Sin. 2009, 29, 2203–2210. [Google Scholar]
- Chen, Z.; Wang, G.; Liu, J.; Xu, W.; Wang, G. Competitive Ability of Two Propagules of Spartina alterniflora With Native Species in the Coastal Wetlands of North Jiangsu. Adv. Mar. Sci. 2012, 30, 380–389. [Google Scholar]
- Wang, H.; Wang, R.Q.; Yu, Y.; Mitchell, M.J.; Zhang, L.J. Soil organic carbon of degraded wetlands treated with freshwater in the Yellow River Delta, China. J. Environ. Manag. 2011, 92, 2628–2633. [Google Scholar] [CrossRef]
- Pennings, S.C.; Grant, M.B.; Bertness, M.D. Plant zonation in low−latitude salt marshes: Disentangling the roles of flooding, salinity and competition. J. Ecol. 2005, 93, 159–167. [Google Scholar] [CrossRef]
- Pennings, S.C.; Selig, E.R.; Houser, L.T.; Bertness, M.D. Geographic variation in positive and negative interactions among salt marsh plants. Ecology 2003, 84, 1527–1538. [Google Scholar] [CrossRef]
- Cui, B.S.; He, Q.A.; An, Y.A. Community Structure and Abiotic Determinants of Salt Marsh Plant Zonation Vary Across Topographic Gradients. Estuaries Coasts 2011, 34, 459–469. [Google Scholar] [CrossRef]
- Davy, A.J.; Brown, M.J.H.; Mossman, H.L.; Grant, A. Colonization of a newly developing salt marsh: Disentangling independent effects of elevation and redox potential on halophytes. J. Ecol. 2011, 99, 1350–1357. [Google Scholar] [CrossRef]
- Wang, C.H.; Lu, M.; Yang, B.; Yang, Q.; Zhang, X.D.; Hara, T.; Li, B. Effects of environmental gradients on the performances of four dominant plants in a Chinese saltmarsh: Implications for plant zonation. Ecol. Res. 2010, 25, 347–358. [Google Scholar] [CrossRef]
- Olff, H.; Leeuw, J.; Bakker, J.P.; Platerink, R.J.; Wijnen, H.J. Vegetation Succession and Herbivory in a Salt Marsh: Changes Induced by Sea Level Rise and Silt Deposition Along an Elevational Gradient. J. Ecol. 1997, 85, 799–814. [Google Scholar] [CrossRef] [Green Version]
- Caçador, I.; Tibério, S.; Cabral, H.N. Species zonation in Corroios salt marsh in the Tagus estuary (Portugal) and its dynamics in the past fifty years. Hydrobiologia 2007, 587, 205–211. [Google Scholar] [CrossRef]
- Travis, J.M.J.; Brooker, R.W.; Clark, E.J.; Dytham, C. The distribution of positive and negative species interactions across environmental gradients on a dual−lattice model. J. Theor. Biol. 2006, 241, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Morzaria-Luna, H.N.; Zedler, J.B. Competitive Interactions between Two Salt Marsh Halophytes across Stress Gradients. Wetlands 2014, 34, 31–42. [Google Scholar]
- Levine, J.M.; Brewer, J.S.; Bertness, M.D. Nutrients, competition and plant zonation in a New England salt marsh. J. Ecol. 1998, 86, 285–292. [Google Scholar] [CrossRef]
- Fountoulakis, M.S.; Sabathianakis, G.; Kritsotakis, I.; Kabourakis, E.M.; Manios, T. Halophytes as vertical−flow constructed wetland vegetation for domestic wastewater treatment. Sci. Total Environ. 2017, 583, 432–439. [Google Scholar] [CrossRef]
- Zhang, C.; Gong, Z.; Qiu, H.; Zhang, Y.; Zhou, D. Mapping typical salt−marsh species in the Yellow River Delta wetland supported by temporal−spatial−spectral multidimensional features. Sci. Total Environ. 2021, 783. [Google Scholar] [CrossRef]
- Li, Y.F.; Wang, C.; Wright, A.; Liu, H.Y.; Zhang, H.B.; Zong, Y. Combination of GF−2 high spatial resolution imagery and land surface factors for predicting soil salinity of muddy coasts. Catena 2021, 202, 10. [Google Scholar] [CrossRef]
- Zhang, R.S.; Shen, Y.M.; Lu, L.Y.; Yan, S.G.; Wang, Y.H.; Li, J.L.; Zhang, Z.L. Formation of Spartina alterniflora salt marshes on the coast of Jiangsu Province, China. Ecol. Eng. 2004, 23, 95–105. [Google Scholar] [CrossRef]
- Ma, Z.W.; Zhang, M.X.; Xiao, R.; Cui, Y.; Yu, F.H. Changes in soil microbial biomass and community composition in coastal wetlands affected by restoration projects in a Chinese delta. Geoderma 2017, 289, 124–134. [Google Scholar] [CrossRef]
- Yang, R.M. Characterization of the salt marsh soils and visible−near−infrared spectroscopy along a chronosequence of Spartina alterniflora invasion in a coastal wetland of Eastern China. Geoderma 2020, 362, 11. [Google Scholar] [CrossRef]
- Huang, L.D.; Zhang, Y.H.; Shi, Y.M.; Liu, Y.B.; Wang, L.; Yan, N. Comparison of phosphorus fractions and phosphatase activities in coastal wetland soils along vegetation zones of Yancheng National Nature Reserve, China. Estuar. Coast. Shelf S 2015, 157, 93–98. [Google Scholar] [CrossRef]
- Ping, Y.M.; Cui, L.J.; Pan, X.; Li, W.; Li, Y.Z.; Kang, X.M.; Song, T.Y.; He, P. Decomposition processes in coastal wetlands: The importance of Suaeda salsa community for soil cellulose decomposition. Pol. J. Ecol. 2018, 66, 217–226. [Google Scholar] [CrossRef]
- Gao, J.H.; Feng, Z.X.; Chen, L.; Wang, Y.P.; Bai, F.; Li, J. The effect of biomass variations of Spartina alterniflora on the organic carbon content and composition of a salt marsh in northern Jiangsu Province, China. Ecol. Eng. 2016, 95, 160–170. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Xu, X.J.; Li, Y.; Huang, L.D.; Xie, X.J.; Dong, J.M.; Yang, S.Q. Effects of Spartina alterniflora invasion and exogenous nitrogen on soil nitrogen mineralization in the coastal salt marshes. Ecol. Eng. 2016, 87, 281–287. [Google Scholar] [CrossRef]
- Bu, N.S.; Qu, J.F.; Li, Z.L.; Li, G.; Zhao, H.; Zhao, B.; Li, B.; Chen, J.K.; Fang, C.M. Effects of Spartina alterniflora Invasion on Soil Respiration in the Yangtze River Estuary, China. PLoS ONE 2015, 10, e0121571. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Q.; Zhang, X.D.; Nie, M.; Fu, C.Z.; Chen, J.K.; Li, B. Exotic Spartina alterniflora provides compatible habitats for native estuarine crab Sesarma dehaani in the Yangtze River estuary. Ecol. Eng. 2008, 34, 57–64. [Google Scholar] [CrossRef]
- Sun, Z.G.; Mou, X.J.; Zhang, D.Y.; Sun, W.L.; Hu, X.Y.; Tian, L.P. Impacts of burial by sediment on decomposition and heavy metal concentrations of Suaeda salsa in intertidal zone of the Yellow River estuary, China. Mar. Pollut. Bull. 2017, 116, 103–112. [Google Scholar] [CrossRef]
- Yang, W.; An, S.Q.; Zhao, H.; Xu, L.Q.; Qiao, Y.J.; Cheng, X.L. Impacts of Spartina alterniflora invasion on soil organic carbon and nitrogen pools sizes, stability, and turnover in a coastal salt marsh of eastern China. Ecol. Eng. 2016, 86, 174–182. [Google Scholar] [CrossRef]
- Yan, J.F.; Wang, L.; Hu, Y.; Tsang, Y.F.; Zhang, Y.N.; Wu, J.H.; Fu, X.H.; Sun, Y. Plant litter composition selects different soil microbial structures and in turn drives different litter decomposition pattern and soil carbon sequestration capability. Geoderma 2018, 319, 194–203. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Ding, W.X.; Cai, Z.C.; Valerie, P.; Han, F.X. Response of methane emission to invasion of Spartina alterniflora and exogenous N deposition in the coastal salt marsh. Atmos. Environ. 2010, 44, 4588–4594. [Google Scholar] [CrossRef]
- Cheng, X.L.; Luo, Y.Q.; Chen, J.Q.; Lin, G.H.; Chen, J.K.; Li, B. Short−term C−4 plant Spartina alterniflora invasions change the soil carbon in C−3 plant−dominated tidal wetlands on a growing estuarine Island. Soil Biol. Biochem. 2006, 38, 3380–3386. [Google Scholar] [CrossRef]
- Yang, W.; Jeelani, N.; Xia, L.; Zhu, Z.H.; Luo, Y.Q.; Cheng, X.L.; An, S.Q. Soil fungal communities vary with invasion by the exotic Spartina alternifolia Loisel. in coastal salt marshes of eastern China. Plant Soil 2019, 442, 215–232. [Google Scholar] [CrossRef]
- Huang, L.B.; Bai, J.H.; Chen, B.; Zhang, K.J.; Huang, C.; Liu, P.P. Two−decade wetland cultivation and its effects on soil properties in salt marshes in the Yellow River Delta, China. Ecol. Inform. 2012, 10, 49–55. [Google Scholar] [CrossRef]
- Zhao, J.; Zhu, Z. Analysis on Diversity of Soil Bacterial Community in Original Coastal Wetland of Yancheng, Jiangsu. Jiangsu Agric. Sci. 2019, 47, 258–261. [Google Scholar]
- Liu, Y. The Assessment of Carbon Storage of Vegetation Zones in the Jiuduan Shoal Wetland. Master’s Thesis, East China Normal University, Shanghai, China, 2013. [Google Scholar]
- Yilan, H.; Lijuan, C.; Chunyi, L.; Wei, L.; Yinru, L. Biologically−Based Availability and Influencing Factors of Soil Phosphorus under Different Vegetation in Coastal Beach Wetlands. Ecol. Environ. Sci. 2019, 28, 1999–2005. [Google Scholar]
- Fan, Q. Carbon, Nitrogen and Phosphorus Content and Ecological Stoichiometry of Spartina Alterniflora in the Tidal Flat Wetland of Jiaozhou Bay. Master’s Thesis, Qingdao University, Qingdao, China, 2019. [Google Scholar]
- Mo, X.; Chen, F.; You, C.; Liu, F. Characteristics and Factors of Soil Enzyme Activity for Different PlantCommunities in Yellow River Delta. Environ. Sci. 2020, 41, 895–904. [Google Scholar]
- Zhang, H. The Characteristics and Mechanism of Landscape Evolution in the Coastal Wetlands under Natural and Human Influence. Ph.D. Thesis, Nanjing Normal University, Nanjing, China, 2013. [Google Scholar]
- Yong−Ming, S.; Hua, Z.; Hui, W.; Yong Mei, L.; Zi Yu, C. Characteristics of halophyte and associated soil along aggradational muddycoasts in Jiangsu Province. Acta Ecol. Sin. 2005, 25, 1–6. [Google Scholar]
- Xu, X.; Zhao, Y.; Zou, X.; Yang, W.; Cao, L.; Cheng, H. The Characteristics of Surficial Sediments Organic Carbon inYancheng Coastal Wetland. J. Nat. Resour. 2014, 29, 1957–1967. [Google Scholar]
- Li, Z.; Zhang, Z.; Li, M.; Zhang, H.; Song, X.; Wu, H. Contents of Organic Carbon and Dissolved Organic Carbon and Characteristics of Functional Group Structure in Surface Soils of Salt Marshes in Yellow River Delta. Wetl. Sci. 2019, 17, 645–650. [Google Scholar]
- Zhang, H.; Liu, H.; Li, Y.; Hou, M. The Coupling Relationship between Soil Eco−Processes and Landscape Evolution under the Natural Conditions in Yancheng Coastal Wetland. J. Nat. Resour. 2013, 28, 63–72. [Google Scholar]
- Yang, D.; Ren, H.; Zhang, Z.; Chen, Y.; Jiang, D. Distribution and Influence factors of soil organic carbon of different land−use types in the Jiangsu coastal areas. J. Subtrop. Resour. Environ. 2016, 11, 46–52. [Google Scholar]
- Zhang, W. Distribution Characteristic and Spatial Heterogeneity of Soil Organic Carbon on the South Coastal of Hangzhou Bay. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2014. [Google Scholar]
- Zhang, W.; Wu, M.; Wang, M.; Shao, X.; Jiang, X.; Zhou, B. Distribution characteristics of organic carbon and its components in soils under different types of vegetation in wetland of Hangzhou Bay. Acta Pedol. Sin. 2014, 51, 1351–1360. [Google Scholar]
- Du, Y.; Xu, Z.; Xie, W.; Zhang, Y. Distribution Characteristics of Phosphorus under Different VegetationCommunities in Salt Marshes of Jiaozhou BayCommunities in Salt Marshes of Jiaozhou Bay. Wetl. Sci. 2016, 14, 415–420. [Google Scholar]
- Zhang, Z. Diversity Survey in Rhizosphere of Diazotroph in the Exotic Invasive Species Spartina Alterniflora and Two Native Species (Phragmites Australis and Scripus Mariqueter) in the Wetlands at Chongming Dongtan in the Yangtze River Estuary. Master’s Thesis, Shanghai Normal University, Shanghai, China, 2012. [Google Scholar]
- Di, L.; Kong, F.; Wang, S.; Li, Y.; Xi, M. Effect of litter decomposition on mineralization of soil organic carbon in the Jiaozhou Bay coastal wetlands. Acta Ecol. Sin. 2019, 39, 8483–8493. [Google Scholar]
- Shen, Z. Effect of Salt on Soil Nitrogen Mineralization in Coastal Wetland of Liaohe Estuary. Master’s Thesis, Shenyang University, Shenyang, China, 2019. [Google Scholar]
- Chen, G. Effect of Spartina alterniflora Invasion on Coastal Wetland Soil Carbon Pool and Stability in Subtropical China. Master’s Thesis, Fujian Normal University, Fuzhou, China, 2018. [Google Scholar]
- Zhang, Y.; Zhang, F.; Zhou, X.; Xie, X.; Wang, X.; Li, Q.; Lei, J. Effects of plant invasion along a Spartina alterniflora chronosequence on organic carbon dynamics in coastal wetland in north Jiangsu. China Environ. Sci. 2011, 31, 271–276. [Google Scholar]
- Yang, X. Effects of Plant Invasion on Soil Caibon Dynamics and CH4 Emissions from Coastal Wetlands. Master’s Thesis, Liaoning University, Shenyang, China, 2019. [Google Scholar]
- Wang, G. The Effects of Salt Marsh Vegetation on Soil Organic Carbon Fractions, Sources and Distribution. Master’s Thesis, Anhui Normal University, Wuhu, China, 2012. [Google Scholar]
- Chen, G.; Gao, D.; Chen, G.; Zeng, C.; Wang, W. Effects of Spartina alterniflora Invasion on Soil Carbon Fractions in Mangrove Wetlands of China. J. Soil Water Conserv. 2017, 31, 249–256. [Google Scholar]
- Jing, B.; Jin-Yu, Y.; Dong-jin, H.; Jin-biao, C.; Ren, W.; Wei-Bin, Y.; Shi-Hong, X.; Dong-Liang, H.; Wei-Wei, L. Effects of Spartina alterniflora invasion in eastern Fujian coastal wetland on the physicochemical properties and enzyme activities of mangrove soil. J. Beijing For. Univ. 2017, 39, 70–77. [Google Scholar]
- Wang, J.; Zhang, W.; Guo, N.; Li, C.; Wang, J. The Key Factor of Impact on Temporal and Spatial Variation of Soil Organic Matter, TN and TP in Coastal Salt Marsh: Tide and Vegetation. Sci. Geogr. Sin. 2016, 36, 247–255. [Google Scholar]
- Xi, M.; Liu, S.; Zhang, Y.; Li, Y.; Kong, F. Leaching Characteristics of Soil Dissovled Organic Carbon in Coastal Wetlands of Jiaozhou Bay. Bulletion Soil Water Conserv. 2019, 39, 16–22. [Google Scholar]
- Ren, L.; Wang, G.; Qiu, L.; Mao, Z.; Liu, J. Morphology and Biomass Distribution of Spartina alterniflora Growing in Different Tidal Flat Habitats in Jiangsu. J. Ecol. Rural Environ. 2010, 26, 220–226. [Google Scholar]
- Xi, M.; Li, M.; Chen, T.; Li, Y.; Kong, F. Nutrient dynamics of litter−soil system during litter decomposition in coastal wetlands of Jiaozhou Bay. Chin. J. Ecol. 2019, 38, 1022–1030. [Google Scholar]
- Chen, Z.; Wang, G.; Liu, J.; Xuan, Y.; Xu, W.; Qiu, L.; Wang, G. Relative competitive ability of Spartina alterniflora patches to native species in tidal zone ecotone of North Jiangsu. Ecol. Environ. Sci. 2011, 20, 1436–1442. (In Chinese) [Google Scholar]
- Ruan, Z. The Relative Importance and Mechanism of Soil Dissimilatory Nitrate Reduction to Ammonium and Denitrification under the Change of Land Use: A Case Study in Chongming Dongtan. Master’s Thesis, Nanjing Forestry University, Nanjing, China, 2018. [Google Scholar]
- Dong, G.; Gao, Y.; Liu, C. Research on characteristics of vegetation distribution pattern and soil factors in the intertidal zone of Zhimai River estuary. Ecol. Sci. 2014, 33, 533–539. [Google Scholar]
- Xu, J.; Su, H.; Yu, P.; Wang, G.; Liu, J. Respirations and Response in Temperature of Salt Marsh Soil in Different Types of Wetlands Along the Coast of Yancheng. J. Ecol. Rural Environ. 2017, 33, 715–721. [Google Scholar]
- Feng, Z.; Gao, J.; Chen, L.; Wang, Y.; Gao, J.; Bai, F. The response of organic carbon content to biomass dynamics in Spartina alterniflora marsh. Acta Ecol. Sin. 2015, 35, 2038–2047. [Google Scholar]
- Ou, W.; Yang, G.; Gao, J. Retention Effect of Wetland for Nitrogen and Phosphorus Nutrients in the Coastal Zone of the Yancheng. Wetl. Sci. 2006, 4, 179–186. [Google Scholar]
- Xi, M.; Xian, X.; Kong, F.; Li, Y.; Yu, X. Soil Quality Evaluation of Bare Flat and Salt Marshes in Jiaozhou Bay Wetlands. Wetl. Sci. 2018, 16, 604–611. [Google Scholar]
- Lu, L. Spatial Distribution and Influencing Factors of the Biomass of Spartina alterniflora in Coastal Wetlands of Zhejiang. Master’s Thesis, Chinese Academy of Forestry, Beijing, China, 2018. [Google Scholar]
- Zhang, H.; Zhen, Y.; Li, Y.; Sun, X. Spatial Heterogeneity of Soil Salinity in Jiangsu Yancheng Wetland National Nature Reserve Rare Birds. Wetl. Sci. 2018, 16, 152–158. [Google Scholar]
- You, C.; Mo, X.; Zhang, S.; Zheng, Y.; Liu, F. The stoichiometric characteristics of different plant communities in the Duliujian River estuary. Chin. J. Appl. Environ. Biol. 2019, 25, 617–625. [Google Scholar]
- Wang, M.; Wu, M.; Shao, X.; Sheng, X. Study on CH4 Emission Fluxes in Hangzhou Bay Coastal Wetland. Soils 2014, 46, 1003–1009. [Google Scholar]
- Wang, M. Study on Methane, Nitrous Oxide and Carbon Dioxide Fluxes and Their Influencing Factors in Hangzhou Bay coastal Wetland. Master’s Thesis, Chinese Academy of Forestry, Beijing, China, 2014. [Google Scholar]
- Liu, W.; Chen, S.; Zheng, C.; Zhu, H.; Huang, L.; Qiu, J.; Huang, X. Temporaland Spatial Variability of Soil Nutrients in Different Vegetation Zones of Yueqing Bay Coastal Wetlands. Chin. J. Soil Sci. 2014, 45, 91–99. [Google Scholar]
- Miao, P.; Xie, W.; Yu, D.; Chen, J.; Gong, J. Vertical distribution and seasonal variation of nitrogen, phosphorus elements in Spartina alterniflora wetland of Jiaozhou Bay, Shandong, China. Chin. J. Appl. Ecol. 2017, 28, 1533–1540. [Google Scholar]






| Soil Factors | Total | Regions | Seasons | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| North | South | Spring | Summer | Autumn | Winter | ||||||||||
| SSc | PAc | SSc | PAc | SSc | PAc | SSc | PAc | SSc | PAc | SSc | PAc | SSc | PAc | ||
| BD | SAc | 0.34 | 0.54 | 0.01 | 0.01 | 0.09 | 0.10 | 0.09 | 0.14 | 0.11 | 0.29 | 0.18 | 0.13 | 0.01 | 0.04 |
| SSc | − | 0.55 | − | 0.75 | − | 0.08 | − | 0.18 | − | 0.25 | − | 0.21 | − | 0.26 | |
| Salinity | SAc | 0.77 | 0.41 | 0.39 | 0.40 | 0.68 | 0.30 | 0.66 | 0.24 | 0.32 | 0.33 | 0.29 | 0.22 | 0.46 | 0.11 |
| SSc | − | 0.42 | − | 0.36 | − | 0.30 | − | 0.25 | − | 0.50 | − | 0.38 | − | 0.12 | |
| pH | SAc | 0.74 | 0.73 | 0.57 | 0.64 | 0.57 | 0.55 | 0.32 | 0.27 | 0.52 | 0.75 | 0.44 | 0.47 | 0.43 | 0.43 |
| SSc | − | 0.86 | − | 0.77 | − | 0.72 | − | 0.52 | − | 0.59 | − | 0.72 | − | 0.85 | |
| TN | SAc | 0.34 | 0.42 | 0.23 | 0.42 | 0.24 | 0.33 | 0.30 | 0.27 | 0.18 | 0.56 | 0.35 | 0.37 | 0.17 | 0.01 |
| SSc | − | 0.70 | − | 0.34 | − | 0.42 | − | 0.61 | − | 0.28 | − | 0.54 | − | 0.05 | |
| TP | SAc | 0.47 | 0.50 | 0.38 | 0.52 | 0.49 | 0.16 | 0.08 | 0.43 | 0.02 | 0.38 | 0.25 | 0.18 | 0.25 | 0.17 |
| SSc | − | 0.47 | − | 0.63 | − | 0.25 | − | 0.08 | − | 0.03 | − | 0.17 | − | 0.23 | |
| Variables | R2 | Adjusted R2 | F | p Values |
|---|---|---|---|---|
| Total | 0.380331 | 0.358199 | ||
| BD | 39.8 | 0.002 ** | ||
| Salinity | 17.5 | 0.002 ** | ||
| pH | 15.3 | 0.002 ** | ||
| TN | 1.3 | 0.26 | ||
| TP | 1 | 0.342 |
| Statistic | Eigenvalues | Explained Variation (Cumulative) | Pseudo-Canonical Correlation | Explained Fitted Variation (Cumulative) |
|---|---|---|---|---|
| Axis 1 | 0.317 | 31.66 | 0.753 | 83.25 |
| Axis 2 | 0.064 | 38.03 | 0.380 | 100 |
| Ranking | Total | Regions | Seasons | ||||
|---|---|---|---|---|---|---|---|
| North | South | Spring | Summer | Autumn | Winter | ||
| 1 | BD (21.7% **) | BD (34.3% **) | BD (15.3% **) | BD (23.2% **) | BD (26.2% **) | BD (35.3% **) | TN (43.4% **) |
| 2 | TN (15.4% **) | TN (6.4% †) | TN (16.4% **) | TP (21.0% **) | TN (16.4% **) | Salinity (15.8% **) | BD (28.3% **) |
| 3 | Salinity (7.1% **) | Salinity (7.8% *) | Salinity (11.6% **) | TN (18.1% **) | TP (8.2% **) | TN (11.6% **) | pH (18.7% †) |
| 4 | TP (2.6% *) | pH (3.9%) | TP (1.6%) | Salinity (12.4% **) | Salinity (4.9% †) | TP (6.5% †) | TP (4.3%) |
| 5 | pH (0.4%) | TP (2.4%) | pH (0.7%) | pH (0.2%) | pH (<0.1%) | pH (0.3%) | Salinity (1.5%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Q.; Zhang, Q.; Liao, A.; Xu, C.; Liu, M. Plant Adaptability and Vegetation Differentiation in the Coastal Beaches of Yellow–Bohai Sea in China. Int. J. Environ. Res. Public Health 2022, 19, 2225. https://doi.org/10.3390/ijerph19042225
Dong Q, Zhang Q, Liao A, Xu C, Liu M. Plant Adaptability and Vegetation Differentiation in the Coastal Beaches of Yellow–Bohai Sea in China. International Journal of Environmental Research and Public Health. 2022; 19(4):2225. https://doi.org/10.3390/ijerph19042225
Chicago/Turabian StyleDong, Qian, Qingqing Zhang, Anbang Liao, Chi Xu, and Maosong Liu. 2022. "Plant Adaptability and Vegetation Differentiation in the Coastal Beaches of Yellow–Bohai Sea in China" International Journal of Environmental Research and Public Health 19, no. 4: 2225. https://doi.org/10.3390/ijerph19042225
APA StyleDong, Q., Zhang, Q., Liao, A., Xu, C., & Liu, M. (2022). Plant Adaptability and Vegetation Differentiation in the Coastal Beaches of Yellow–Bohai Sea in China. International Journal of Environmental Research and Public Health, 19(4), 2225. https://doi.org/10.3390/ijerph19042225