Arsenic Release from Soil Induced by Microorganisms and Environmental Factors
Abstract
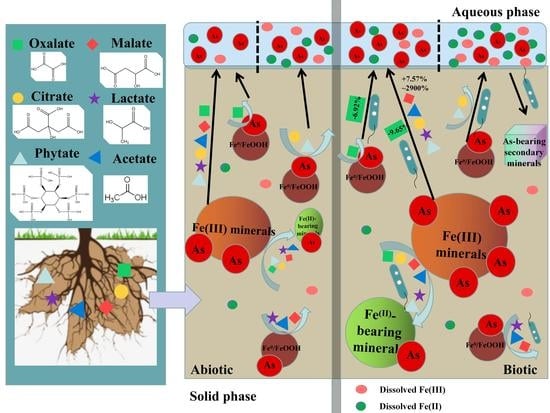
:1. Introduction
2. Materials and Methods
2.1. Soil Sampling and Pretreatment
2.2. Microcosm Experiments
2.2.1. Experiment I (LMWOASs)
2.2.2. Experiment II (Phosphates)
2.3. Geochemical Analysis
2.4. Quality Assurance/Quality Control (QA/QC) and Statistical Analysis
3. Results and Discussion
3.1. Arsenic Release under LMWOAS Treatments in Unrestored Soil
3.2. Arsenic Release under LMWOAS Treatments in Restored Soil
3.3. Effect of Phosphate on as Release in Restored and Unrestored Soils
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wan, X.; Lei, M.; Chen, T. Review on remediation technologies for arsenic-contaminated soil. Front. Environ. Sci. Eng. 2019, 14, 24. [Google Scholar] [CrossRef]
- Gong, Y.; Zhao, D.; Wang, Q. An overview of field-scale studies on remediation of soil contaminated with heavy metals and metalloids: Technical progress over the last decade. Water Res. 2018, 147, 440–460. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-Y.; Yan, X.-L.; Yang, S. Stabilizing effects of Fe-Ce oxide on soil As(V) and P. Environ. Sci. 2019, 40, 3785–3791. [Google Scholar]
- Wu, J.; Li, Z.; Huang, D.; Liu, X.; Tang, C.; Parikh, S.J.; Xu, J. A novel calcium-based magnetic biochar is effective in stabilization of arsenic and cadmium co-contamination in aerobic soils. J. Hazard. Mater. 2020, 387, 122010. [Google Scholar] [CrossRef]
- Zhai, W.; Dai, Y.; Zhao, W.; Yuan, H.; Qiu, D.; Chen, J.; Gustave, W.; Maguffin, S.C.; Chen, Z.; Liu, X.; et al. Simultaneous immobilization of the cadmium, lead and arsenic in paddy soils amended with titanium gypsum. Environ. Pollut. 2020, 258, 113790. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Cai, D.; Tu, S. Arsenite simultaneous sorption and oxidation by natural ferruginous manganese ores with various ratios of Mn/Fe. Chem. Eng. J. 2020, 382, 123040. [Google Scholar] [CrossRef]
- Zheng, Q.; Hou, J.; Hartley, W.; Ren, L.; Wang, M.; Tu, S.; Tan, W. As(III) adsorption on Fe-Mn binary oxides: Are Fe and Mn oxides synergistic or antagonistic for arsenic removal? Chem. Eng. J. 2020, 389, 124470. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, T.; White, J.C.; Lin, D. A new strategy using nanoscale zero-valent iron to simultaneously promote remediation and safe crop production in contaminated soil. Nat. Nanotechnol. 2021, 16, 197–205. [Google Scholar] [CrossRef]
- Hou, Q.; Han, D.; Zhang, Y.; Han, M.; Huang, G.; Xiao, L. The bioaccessibility and fractionation of arsenic in anoxic soils as a function of stabilization using low-cost Fe/Al-based materials: A long-term experiment. Ecotoxicol. Environ. Saf. 2020, 191, 110210. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Wu, J.; Xu, Y.; Wang, F.; Tang, X.; Xu, J.; Ok, Y.S.; Meng, J.; Liu, X. Zeolite-supported nanoscale zero-valent iron for immobilization of cadmium, lead, and arsenic in farmland soils: Encapsulation mechanisms and indigenous microbial responses. Environ. Pollut. 2020, 260, 114098. [Google Scholar] [CrossRef]
- Allegretta, I.; Porfido, C.; Martin, M.; Barberis, E.; Terzano, R.; Spagnuolo, M. Characterization of As-polluted soils by laboratory X-ray-based techniques coupled with sequential extractions and electron microscopy: The case of Crocette gold mine in the Monte Rosa mining district (Italy). Environ. Sci. Pollut. Res. 2018, 25, 25080–25090. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Jeong, B.; Nam, K. Evaluation of the effectiveness of in situ stabilization in the field aged arsenic-contaminated soil: Chemical extractability and biological response. J. Hazard. Mater. 2019, 367, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Corsini, A.; Cavalca, L.; Zaccheo, P.; Crippa, L.; Andreoni, V. Influence of microorganisms on arsenic mobilization and speciation in a submerged contaminated soil: Effects of citrate. Appl. Soil Ecol. 2011, 49, 99–106. [Google Scholar] [CrossRef]
- Zhang, Z.; Moon, H.S.; Myneni, S.C.B.; Jaffé, P.R. Phosphate enhanced abiotic and biotic arsenic mobilization in the wetland rhizosphere. Chemosphere 2017, 187, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Xie, Z.; Wang, J.; Liu, D.; Zhong, Z. Bacterially mediated release and mobilization of As/Fe coupled to nitrate reduction in a sediment environment. Ecotoxicol. Environ. Saf. 2021, 208, 111478. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Zhao, F.; Liu, J.; Frost, R.L. The As behavior of natural arsenical-containing colloidal ferric oxyhydroxide reacted with sulfate reducing bacteria. Chem. Eng. J. 2018, 332, 183–191. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.H. Impact of competitive adsorption on microbial arsenate reduction at the water-goethite interface. Appl. Geochem. 2018, 88, 59–67. [Google Scholar] [CrossRef]
- Huang, J.-H. Characterising microbial reduction of arsenate sorbed to ferrihydrite and its concurrence with iron reduction. Chemosphere 2018, 194, 49–56. [Google Scholar] [CrossRef]
- Yamamura, S.; Sudo, T.; Watanabe, M.; Tsuboi, S.; Soda, S.; Ike, M.; Amachi, S. Effect of extracellular electron shuttles on arsenic-mobilizing activities in soil microbial communities. J. Hazard. Mater. 2018, 342, 571–578. [Google Scholar] [CrossRef]
- Zhalnina, K.; Louie, K.B.; Hao, Z.; Mansoori, N.; da Rocha, U.N.; Shi, S.; Cho, H.; Karaoz, U.; Loque, D.; Bowen, B.P.; et al. Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nat. Microbiol. 2018, 3, 470–480. [Google Scholar] [CrossRef] [Green Version]
- Van Hees, P.A.W.; Vinogradoff, S.I.; Edwards, A.C.; Godbold, D.L.; Jones, D.L. Low molecular weight organic acid adsorption in forest soils: Effects on soil solution concentrations and biodegradation rates. Soil Biol. Biochem. 2003, 35, 1015–1026. [Google Scholar] [CrossRef]
- Jones, D.L.; Dennis, P.G.; Owen, A.G.; van Hees, P.A.W. Organic acid behavior in soils-misconceptions and knowledge gaps. Plant Soil 2003, 248, 31–41. [Google Scholar] [CrossRef]
- Tu, S.X.; Ma, L.; Luongo, T. Root exudates and arsenic accumulation in arsenic hyperaccumulating Pteris vittata and non-hyperaccumulating Nephrolepis exaltata. Plant Soil 2004, 258, 9–19. [Google Scholar] [CrossRef]
- Gerke, J.; Romer, W.; Jungk, A. The excretion of citric and malic acid by proteoid roots of Lupinus albus L.; effects on soil solution concentrations of phosphate, iron, and aluminum in the proteoid rhizosphere in samples of an oxisol and a luvisol. Z. Med. Phys. 1994, 157, 289–294. [Google Scholar] [CrossRef]
- Liu, L.; Yang, Y.-P.; Duan, G.-L.; Wang, J.; Tang, X.-J.; Zhu, Y.-G. The chemical-microbial release and transformation of arsenic induced by citric acid in paddy soil. J. Hazard. Mater. 2022, 421, 126731. [Google Scholar] [CrossRef]
- Lee, J.-C.; Kim, E.J.; Baek, K. Synergistic effects of the combination of oxalate and ascorbate on arsenic extraction from contaminated soils. Chemosphere 2017, 168, 1439–1446. [Google Scholar] [CrossRef]
- Mei, K.; Liu, J.; Shi, R.; Guo, X.; Lu, H.; Yan, C. The migrated behavior and bioavailability of arsenic in mangrove sediments affected by pH and organic acids. Mar. Pollut. Bull. 2020, 159, 111480. [Google Scholar] [CrossRef]
- Xu, Y.; Wan, L.; Wang, K.; Liu, C.; Zhang, J. Enhanced mobilization of arsenic from tailing soil by four types of low molecular weight organic acids with different functional groups. J. Soils Sediments 2021, 21, 3834–3844. [Google Scholar] [CrossRef]
- Ji, Y.; Luo, W.; Lu, G.; Fan, C.; Tao, X.; Ye, H.; Xie, Y.; Shi, Z.; Yi, X.; Dang, Z. Effect of phosphate on amorphous iron mineral generation and arsenic behavior in paddy soils. Sci. Total Environ. 2019, 657, 644–656. [Google Scholar] [CrossRef]
- Deng, Y.; Weng, L.; Li, Y.; Chen, Y.; Ma, J. Redox-dependent effects of phosphate on arsenic speciation in paddy soils. Environ. Pollut. 2020, 264, 114783. [Google Scholar] [CrossRef]
- Yan, G.; Chen, X.; Du, S.; Deng, Z.; Wang, L.; Chen, S. Genetic mechanisms of arsenic detoxification and metabolism in bacteria. Curr. Genet. 2019, 65, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xie, Z.; Wei, X.; Chen, M.; Luo, Y.; Wang, Y. An indigenous bacterium Bacillus XZM for phosphate enhanced transformation and migration of arsenate. Sci. Total Environ. 2020, 719, 137183. [Google Scholar] [CrossRef] [PubMed]
- Onireti, O.O.; Lin, C. Mobilization of soil-borne arsenic by three common organic acids: Dosage and time effects. Chemosphere 2016, 147, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Luo, T.; Zhong, S.; Zhou, F.; Zhang, Y.; Ma, Y.; Fu, Q. Long-term effects of low-molecular-weight organic acids on remobilization of Cd, Cr, Pb, and As in alkaline coastal wetland soil. Env. Pollut. Bioavail. 2021, 33, 266–277. [Google Scholar] [CrossRef]
- Onireti, O.O.; Lin, C.; Qin, J. Combined effects of low-molecular-weight organic acids on mobilization of arsenic and lead from multi-contaminated soils. Chemosphere 2017, 170, 161–168. [Google Scholar] [CrossRef]
- Nworie, O.E.; Qin, J.; Lin, C. Differential effects of low-molecular-weight organic acids on the mobilization of soil-borne arsenic and trace metals. Toxics 2017, 5, 18. [Google Scholar] [CrossRef] [Green Version]
- Vitkova, M.; Komarek, M.; Tejnecky, V.; Sillerova, H. Interactions of nano-oxides with low-molecular-weight organic acids in a contaminated soil. J. Hazard. Mater. 2015, 293, 7–14. [Google Scholar] [CrossRef]
- Wang, S.; Mulligan, C.N. Effects of three low-molecular-weight organic acids (LMWOAs) and pH on the mobilization of arsenic and heavy metals (Cu, Pb, and Zn) from mine tailings. Environ. Geochem. Health 2013, 35, 111–118. [Google Scholar] [CrossRef]
- Mei, K.; Wu, G.; Liu, J.; Jiajia, W.; Hong, H.; Lu, H.; Yan, C. Dynamics of low-molecular-weight organic acids for the extraction and sequestration of arsenic species and heavy metals using mangrove sediments. Chemosphere 2022, 286, 131820. [Google Scholar] [CrossRef]
- Chen, Z.; Dong, G.; Gong, L.; Li, Q.; Wang, Y. The role of low-molecular-weight organic carbons in facilitating the mobilization and biotransformation of As(V)/Fe(III) from a realgar tailing mine soil. Geomicrobiol. J. 2018, 35, 555–563. [Google Scholar] [CrossRef]
- Chen, Z.; Li, D.; Luo, X. Research on arsenic form in the gold mine tailings by different leaching processes. Rock Miner. Anal. 2014, 33, 363–368. [Google Scholar]
- Luo, X.M. Case Study on the Migration and Transform of Arsenic in Aquatic Environment Caused by Gold Mining—Dandong, Liaoning. Ph.D. Thesis, China University of Geosciences, Beijing, China, 2007. [Google Scholar]
- Wang, Y.; Zeng, X.; Lu, Y.; Bai, L.; Su, S.; Wu, C. Dynamic arsenic aging processes and their mechanisms in nine types of Chinese soils. Chemosphere 2017, 187, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, S.; Ike, M.; Fujita, M. Dissimilatory arsenate reduction by a facultative anaerobe, Bacillus sp. strain SF-1. J. Biosci. Bioeng. 2003, 96, 454–460. [Google Scholar] [CrossRef]
- Yamamura, S.; Kurasawa, H.; Kashiwabara, Y.; Hori, T.; Aoyagi, T.; Nakajima, N.; Amachi, S. Soil microbial communities involved in reductive dissolution of arsenic from arsenate-laden minerals with different carbon sources. Environ. Sci. Technol. 2019, 53, 12398–12406. [Google Scholar] [CrossRef]
- Fredrickson, J.K.; Gorby, Y.A. Environmental processes mediated by iron-reducing bacteria. Curr. Opin. Biotechnol. 1996, 7, 287–294. [Google Scholar] [CrossRef]
- Liu, Y.; Li, F.-B.; Xia, W.; Xu, J.-M.; Yu, X.-S. Association between ferrous iron accumulation and pentachlorophenol degradation at the paddy soil-water interface in the presence of exogenous low-molecular-weight dissolved organic carbon. Chemosphere 2013, 91, 1547–1555. [Google Scholar] [CrossRef]
- Gao, S.-J.; Cao, W.-D.; Gao, J.-S.; Huang, J.; Bai, J.-S.; Zeng, N.-H.; Chang, D.-N.; Shimizu, K. Effects of long-term application of different green manures on ferric iron reduction in a red paddy soil in Southern China. J. Integr. Agric. 2017, 16, 959–966. [Google Scholar] [CrossRef]
- Song, J.; Yang, J.; Cui, X. Effects of low molecular-weight organic acids/sallts on availability of Lead, Zinc and Arsenic in mixed metal-polluted soil. J. Soil. Water Conserv. 2010, 24, 108–112,118. [Google Scholar]
- Wang, Y.; Zhang, G.; Wang, H.; Cheng, Y.; Liu, H.; Jiang, Z.; Li, P.; Wang, Y. Effects of different dissolved organic matter on microbial communities and arsenic mobilization in aquifers. J. Hazard. Mater. 2021, 411, 125146. [Google Scholar] [CrossRef]
- De Araujo, T.O.; Isaure, M.-P.; Alchoubassi, G.; Bierla, K.; Szpunar, J.; Trcera, N.; Chay, S.; Alcon, C.; da Silva, L.C.; Curie, C.; et al. Paspalum urvillei and Setaria parviflora, two grasses naturally adapted to extreme iron-rich environments. Plant Physiol. Biochem. 2020, 151, 144–156. [Google Scholar] [CrossRef]
- Wu, F.; Xu, F.; Ma, X.; Luo, W.; Lou, L.; Wong, M.H. Do arsenate reductase activities and oxalate exudation contribute to variations of arsenic accumulation in populations of Pteris vittata? J. Soils Sediments 2018, 18, 3177–3185. [Google Scholar] [CrossRef]
- Mei, K.; Liu, J.; Fan, J.; Guo, X.; Wu, J.; Zhou, Y.; Lu, H.; Yan, C. Low-level arsenite boosts rhizospheric exudation of low-molecular-weight organic acids from mangrove seedlings (Avicennia marina): Arsenic phytoextraction, removal, and detoxification. Sci. Total Environ. 2021, 775, 145685. [Google Scholar] [CrossRef]
- Chen, C.; Dynes, J.J.; Wang, J.; Sparks, D.L. Properties of Fe-organic matter associations via coprecipitation versus adsorption. Environ. Sci. Technol. 2014, 48, 13751–13759. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, L.; Meng, J.; Liu, X.; Xu, J.; Wang, F.; Brookes, P. Zeolite-supported nanoscale zero-valent iron: New findings on simultaneous adsorption of Cd(II), Pb(II), and As(III) in aqueous solution and soil. J. Hazard. Mater. 2018, 344, 1–11. [Google Scholar] [CrossRef]
- Kim, E.J.; Baek, K. Enhanced reductive extraction of arsenic from contaminated soils by a combination of dithionite and oxalate. J. Hazard. Mater. 2015, 284, 19–26. [Google Scholar] [CrossRef]
- Hong, J.; Liu, L.; Tan, W.; Qiu, G. Arsenic release from arsenopyrite oxidative dissolution in the presence of citrate under UV irradiation. Sci. Total Environ. 2020, 726, 138429. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Fu, J.W.; Guan, D.X.; Cao, Y.; Luo, J.; Rathinasabapathi, B.; Chen, Y.S.; Ma, L.Q. Arsenic induced phytate exudation, and promoted FeAsO4 dissolution and plant growth in As-hyperaccumulator Pteris vittata. Environ. Sci. Technol. 2016, 50, 9070–9077. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, N.; Sun, B.; Su, S.; Wang, Y.; Zhang, Y.; Wu, C.; Zeng, X. Contradictory tendency of As(V) releasing from Fe–As complexes: Influence of organic and inorganic anions. Chemosphere 2022, 286, 131469. [Google Scholar] [CrossRef]
- Cai, X.; Yin, N.; Wang, P.; Du, H.; Liu, X.; Cui, Y. Arsenate-reducing bacteria-mediated arsenic speciation changes and redistribution during mineral transformations in arsenate-associated goethite. J. Hazard. Mater. 2020, 398, 122886. [Google Scholar] [CrossRef]
- Strawn, D.G. Review of interactions between phosphorus and arsenic in soils from four case studies. Geochem. Trans. 2018, 19, 10. [Google Scholar] [CrossRef] [Green Version]
- Cai, R.; Wang, X.; Ji, X.; Peng, B.; Tan, C.; Huang, X. Phosphate reclaim from simulated and real eutrophic water by magnetic biochar derived from water hyacinth. J. Environ. Manag. 2017, 187, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Wang, X.; Xing, B. Removal of labile arsenic from flooded paddy soils with a novel extractive column loaded with quartz-supported nanoscale zero-valent iron. Environ. Pollut. 2019, 255, 113249. [Google Scholar] [CrossRef] [PubMed]









| Property | Value/Content |
|---|---|
| pH | 7.61 |
| Organic matter/(mg/kg) | 10,500 |
| Total C/(mg/kg) | 14,500 |
| Total P/(mg/kg) | 549 |
| Total S/(mg/kg) | 3200 |
| Total N/(mg/kg) | 636 |
| Total Fe/(mg/kg) | 33,400 |
| Total As/(mg/kg) | 1944 |
| Available As/(mg/kg) | 35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, Y.; Luo, X.; Guan, X.; Zhao, J.; Tan, Y.; Shi, X.; Luo, M.; Han, X. Arsenic Release from Soil Induced by Microorganisms and Environmental Factors. Int. J. Environ. Res. Public Health 2022, 19, 4512. https://doi.org/10.3390/ijerph19084512
Yin Y, Luo X, Guan X, Zhao J, Tan Y, Shi X, Luo M, Han X. Arsenic Release from Soil Induced by Microorganisms and Environmental Factors. International Journal of Environmental Research and Public Health. 2022; 19(8):4512. https://doi.org/10.3390/ijerph19084512
Chicago/Turabian StyleYin, Yitong, Ximing Luo, Xiangyu Guan, Jiawei Zhao, Yuan Tan, Xiaonan Shi, Mingtao Luo, and Xiangcai Han. 2022. "Arsenic Release from Soil Induced by Microorganisms and Environmental Factors" International Journal of Environmental Research and Public Health 19, no. 8: 4512. https://doi.org/10.3390/ijerph19084512
APA StyleYin, Y., Luo, X., Guan, X., Zhao, J., Tan, Y., Shi, X., Luo, M., & Han, X. (2022). Arsenic Release from Soil Induced by Microorganisms and Environmental Factors. International Journal of Environmental Research and Public Health, 19(8), 4512. https://doi.org/10.3390/ijerph19084512