Properties of the Omicron Variant of SARS-CoV-2 Affect Public Health Measure Effectiveness in the COVID-19 Epidemic
Abstract
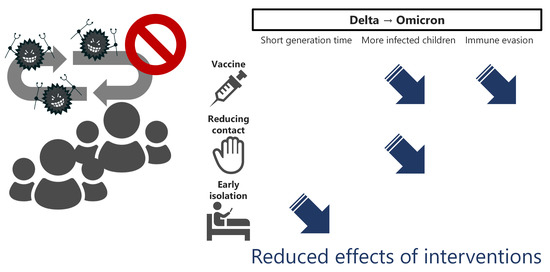
:1. Introduction
2. Materials and Methods
2.1. Simulation Model
2.2. Infection Spread
2.3. Properties of the Omicron Variant
2.4. Interventions
2.5. Data Availability
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haug, N.; Geyrhofer, L.; Londei, A.; Dervic, E.; Desvars-Larrive, A.; Loreto, V.; Pinior, B.; Thurner, S.; Klimek, P. Ranking the effectiveness of worldwide COVID-19 government interventions. Nat. Hum. Behav. 2020, 4, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Mindermann, S.; Rogers-Smith, C.; Leech, G.; Snodin, B.; Ahuja, J.; Sandbrink, J.B.; Monrad, J.T.; Altman, G.; Dhaliwal, G.; et al. Understanding the effectiveness of government interventions against the resurgence of COVID-19 in Europe. Nat. Commun. 2021, 12, 5820. [Google Scholar] [CrossRef]
- Contreras, S.; Dehning, J.; Loidolt, M.; Zierenberg, J.; Spitzner, F.P.; Urrea-Quintero, J.H.; Mohr, S.B.; Wilczek, M.; Wibral, M.; Priesemann, V. The challenges of containing SARS-CoV-2 via test-trace-and-isolate. Nat. Commun. 2021, 12, 378. [Google Scholar] [CrossRef]
- Oshitani, H. Cluster-based approach to Coronavirus Disease 2019 (COVID-19) response in Japan—February–April 2020. Jpn. J. Infect. Dis. 2020, 73, 491–493. [Google Scholar] [CrossRef] [PubMed]
- Furuse, Y.; Sando, E.; Tsuchiya, N.; Miyahara, R.; Yasuda, I.; Ko, Y.K.; Saito, M.; Morimoto, K.; Imamura, T.; Shobugawa, Y.; et al. Clusters of coronavirus disease in communities, Japan, January–April 2020. Emerg. Infect. Dis. 2020, 26, 2176–2179. [Google Scholar] [CrossRef] [PubMed]
- Tregoning, J.S.; Flight, K.E.; Higham, S.L.; Wang, Z.; Pierce, B.F. Progress of the COVID-19 vaccine effort: Viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat. Rev. Immunol. 2021, 21, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Furuse, Y. Simulation of future COVID-19 epidemic by vaccination coverage scenarios in Japan. J. Glob. Health 2021, 11, 05025. [Google Scholar] [CrossRef]
- Tao, K.; Tzou, P.L.; Nouhin, J.; Gupta, R.K.; de Oliveira, T.; Kosakovsky Pond, S.L.; Fera, D.; Shafer, R.W. The biological and clinical significance of emerging SARS-CoV-2 variants. Nat. Rev. Genet. 2021, 22, 757–773. [Google Scholar] [CrossRef]
- Karim, S.S.A.; Karim, Q.A. Omicron SARS-CoV-2 variant: A new chapter in the COVID-19 pandemic. Lancet 2021, 398, 2126–2128. [Google Scholar] [CrossRef]
- Viana, R.; Moyo, S.; Amoako, D.G.; Tegally, H.; Scheepers, C.; Althaus, C.L.; Anyaneji, U.J.; Bester, P.A.; Boni, M.F.; Chand, M.; et al. Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in southern Africa. Nature 2022, 603, 679–686. [Google Scholar] [CrossRef]
- Abbott, S.; Sherratt, K.; Gerstung, M.; Funk, S. Estimating Generation Time of Omicron-COVID-19. Available online: http://sonorouschocolate.com/covid19/index.php?title=Estimating_Generation_Time_Of_Omicron (accessed on 17 March 2022).
- Selby, A. Estimation of the Test to Test Distribution as a Proxy for Generation Interval Distribution for the Omicron Variant in England. Available online: https://epiforecasts.io/omicron-sgtf-forecast/generation-time (accessed on 17 March 2022).
- Roser, M. Our World in Data. 2014. Available online: https://ourworldindata.org/ (accessed on 20 February 2021).
- Statistics Bureau Ministry of Internal Affairs and Communications. Population of Japan. Available online: https://www.stat.go.jp/data/jinsui/1.html#kijun (accessed on 23 March 2022).
- Ministry of Health Labour and Welfare. Visualizing the Data: Information on COVID-19 Infections. Available online: https://covid19.mhlw.go.jp/en/ (accessed on 17 March 2022).
- Meng, B.; Abdullahi, A.; Ferreira, I.A.; Goonawardane, N.; Saito, A.; Kimura, I.; Yamasoba, D.; Gerber, P.P.; Fatihi, S.; Rathore, S.; et al. Altered TMPRSS2 usage by SARS-CoV-2 Omicron impacts infectivity and fusogenicity. Nature 2022, 603, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.; DeWitt, P.E.; Russell, S.; Sanchez-Pinto, L.N.; Haendel, M.A.; Moffitt, R.; Bennett, T.D. Acute upper airway disease in children with the omicron (B.1.1.529) variant of SARS-CoV-2: A report from the National COVID Cohort Collaborative (N3C). medRxiv 2022. [Google Scholar] [CrossRef]
- Prime Minister’s Office of Japan. about COVID-19 Vaccination. Available online: https://www.kantei.go.jp/jp/headline/kansensho/vaccine.html (accessed on 17 March 2022). (In Japanese)
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.M.; et al. Covid-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 2022. [Google Scholar] [CrossRef] [PubMed]
- Collie, S.; Champion, J.; Moultrie, H.; Bekker, L.G.; Gray, G. Effectiveness of BNT162b2 Vaccine against Omicron Variant in South Africa. N. Engl. J. Med. 2022, 386, 494–496. [Google Scholar] [CrossRef]
- Furuse, Y.; Tsuchiya, N.; Miyahara, R.; Yasuda, I.; Sando, E.; Ko, Y.K.; Imamura, T.; Morimoto, K.; Imamura, T.; Shobugawa, Y.; et al. COVID-19 case-clusters and transmission chains in the communities in Japan. J. Infect. 2022, 84, 248–288. [Google Scholar] [CrossRef] [PubMed]
- Madewell, Z.J.; Yang, Y.; Longini, I.M.; Halloran, M.E.; Dean, N.E. Household Transmission of SARS-CoV-2: A Systematic Review and Meta-analysis. JAMA Netw. Open 2020, 3, e2031756. [Google Scholar] [CrossRef] [PubMed]
- Woodworth, K.R.; Moulia, D.; Collins, J.P.; Hadler, S.C.; Jones, J.M.; Reddy, S.C.; Chamberland, M.; Campos-Outcalt, D.; Morgan, R.L.; Brooks, O.; et al. The Advisory Committee on Immunization Practices’ Interim Recommendation for Use of Pfizer-BioNTech COVID-19 Vaccine in Children Aged 5–11 Years—United States, November 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1579–1583. [Google Scholar] [CrossRef]
- Kim, C.; Yee, R.; Bhatkoti, R.; Carranza, D.; Henderson, D.; Kuwabara, S.A.; Trinidad, J.P.; Radesky, S.; Cohen, A.; Vogt, T.M.; et al. COVID-19 Vaccine Provider Access and Vaccination Coverage Among Children Aged 5–11 Years—United States, November 2021–January 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 378–383. [Google Scholar] [CrossRef]
- Stein, M.; Ashkenazi-Hoffnung, L.; Greenberg, D.; Dalal, I.; Livni, G.; Chapnick, G.; Stein-Zamir, C.; Ashkenazi, S.; Hecht-Sagie, L.; Grossman, Z. The Burden of COVID-19 in Children and Its Prevention by Vaccination: A Joint Statement of the Israeli Pediatric Association and the Israeli Society for Pediatric Infectious Diseases. Vaccines 2022, 10, 81. [Google Scholar] [CrossRef]
- Cabinet Secretariat Government of Japan. Measures to Be Taken Based on the Basic Response Policy. Available online: https://corona.go.jp/en/emergency/ (accessed on 17 March 2022).
- Opel, D.J.; Diekema, D.S.; Ross, L.F. Should We Mandate a COVID-19 Vaccine for Children? JAMA Pediatrics 2021, 175, 125–126. [Google Scholar] [CrossRef]
- Klass, P.; Ratner, A.J. Vaccinating Children against COVID-19—The Lessons of Measles. N. Engl. J. Med. 2021, 384, 589–591. [Google Scholar] [CrossRef] [PubMed]
- Brusa, M.; Barilan, Y.M. Voluntary COVID-19 vaccination of children: A social responsibility. J. Med. Ethics 2021, 47, 543–546. [Google Scholar] [CrossRef] [PubMed]
- Pei, S.; Kandula, S.; Shaman, J. Differential effects of intervention timing on COVID-19 spread in the United States. Sci. Adv. 2020, 6, eabd6370. [Google Scholar] [CrossRef] [PubMed]
- Nishi, A.; Dewey, G.; Endo, A.; Neman, S.; Iwamoto, S.K.; Ni, M.Y.; Tsugawa, Y.; Iosifidis, G.; Smith, J.D.; Young, S.D. Network interventions for managing the COVID-19 pandemic and sustaining economy. Proc. Natl. Acad. Sci. USA 2020, 117, 30285–30294. [Google Scholar] [CrossRef] [PubMed]
- Sonabend, R.; Whittles, L.K.; Imai, N.; Perez-Guzman, P.N.; Knock, E.S.; Rawson, T.; Gaythorpe, K.A.; Djaafara, B.A.; Hinsley, W.; FitzJohn, R.G.; et al. Non-pharmaceutical interventions, vaccination, and the SARS-CoV-2 delta variant in England: A mathematical modelling study. Lancet 2021, 398, 1825–1835. [Google Scholar] [CrossRef]
- UK Health Security Agency. SARS-CoV-2 Variants of Concern and Variants under Investigation in England: Technical Briefing 40. 2022. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1067672/Technical-Briefing-40-8April2022.pdf (accessed on 23 March 2022).



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furuse, Y. Properties of the Omicron Variant of SARS-CoV-2 Affect Public Health Measure Effectiveness in the COVID-19 Epidemic. Int. J. Environ. Res. Public Health 2022, 19, 4930. https://doi.org/10.3390/ijerph19094930
Furuse Y. Properties of the Omicron Variant of SARS-CoV-2 Affect Public Health Measure Effectiveness in the COVID-19 Epidemic. International Journal of Environmental Research and Public Health. 2022; 19(9):4930. https://doi.org/10.3390/ijerph19094930
Chicago/Turabian StyleFuruse, Yuki. 2022. "Properties of the Omicron Variant of SARS-CoV-2 Affect Public Health Measure Effectiveness in the COVID-19 Epidemic" International Journal of Environmental Research and Public Health 19, no. 9: 4930. https://doi.org/10.3390/ijerph19094930
APA StyleFuruse, Y. (2022). Properties of the Omicron Variant of SARS-CoV-2 Affect Public Health Measure Effectiveness in the COVID-19 Epidemic. International Journal of Environmental Research and Public Health, 19(9), 4930. https://doi.org/10.3390/ijerph19094930