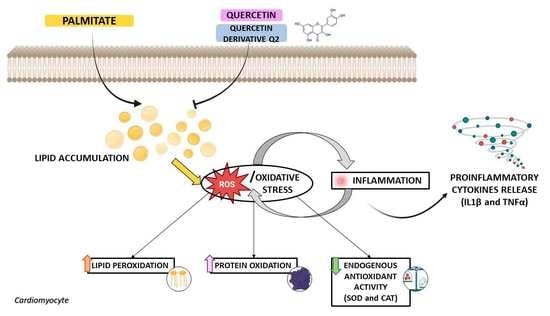
Quercetin and Its Derivative Counteract Palmitate-Dependent Lipotoxicity by Inhibiting Oxidative Stress and Inflammation in Cardiomyocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cell Culture
2.3. Cell Viability Assay
2.4. Lactate Dehydrogenase (LDH) Evaluation
2.5. Oil Red O Staining
2.6. Assessment of Malondialdehyde (MDA) Concentration
2.7. Measurement of Protein Carbonyl Content
2.8. Evaluation of Superoxide Dismutase (SOD) Enzyme Activity
2.9. Catalase (CAT) Activity Assay
2.10. Detection of Intracellular Reactive Oxygen Species (ROS)
2.11. ELISAs Assay for the Assessment of Pro-Inflammatory Cytokines
2.12. Statistical Analysis
3. Results
3.1. Effect of QUE and Its Derivative, Q2, on Cell Viability of H9c2 Cardiomyocytes Exposed to PA
3.2. Action of QUE against PA-Dependent Cytotoxic and Lipotoxic Damage in Cardiomyocytes
3.3. Effects of QUE on PA-Provoked Oxidative Stress and Impaired SOD and CAT Enzymatic Activities in H9c2 Cardiomyocytes
3.4. Effect of QUE against PA-Dependent Inflammation in Cardiomyocytes
3.5. Effects of Q2 against PA-Provoked Cytotoxicity and Intracellular Lipid Accumulation in H9c2 Cardiomyocytes
3.6. Action of Q2 against PA-Dependent Oxidative Stress and Inflammation in H9c2 Cardiomyocytes
4. Discussion
4.1. QUE and Its Derivate, Q2, Counteract PA-Dependent Cardiomyocyte Death
4.2. QUE Relieves PA-Induced Cytotoxicity and Accumulation of Lipid Droplets in Cardiomyocytes
4.3. QUE Reduces PA-Induced Oxidative Stress and ROS Overproduction and Improves the Endogenous Antioxidant Defences
4.4. QUE Mitigates PA-Provoked Inflammatory Response, Reducing the Release of Proinflammatory Cytokines
4.5. The QUE-Derivative Q2 Counteracts PA-Dependent Lipotoxicity by Mitigating Cytotoxicity, the Accumulation of Intracellular Lipids, Oxidative Stress, and Inflammation in Cardiomyocytes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ren, J.; Wu, N.N.; Wang, S.; Sowers, J.R.; Zhang, Y. Obesity cardiomyopathy: Evidence, mechanisms, and therapeutic implications. Physiol. Rev. 2021, 101, 1745–1807. [Google Scholar] [CrossRef] [PubMed]
- Gadde, K.M.; Martin, C.K.; Berthoud, H.R.; Heymsfield, S.B. Obesity: Pathophysiology and Management. J. Am. Coll. Cardiol. 2018, 71, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sowers, J.R.; Ren, J. Pathophysiological insights into cardiovascular health in metabolic syndrome. Exp. Diabetes Res. 2012, 2012, 320534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unger, R.H.; Orci, L. Lipoapoptosis: Its mechanism and its diseases. Biochim. Biophys. Acta 2002, 1585, 202–212. [Google Scholar] [CrossRef]
- McGavock, J.M.; Lingvay, I.; Zib, I.; Tillery, T.; Salas, N.; Unger, R.; Levine, B.D.; Raskin, P.; Victor, R.G.; Szczepaniak, L.S. Cardiac steatosis in diabetes mellitus: A 1H-magnetic resonance spectroscopy study. Circulation 2007, 116, 1170–1175. [Google Scholar] [CrossRef] [Green Version]
- Pasqua, T.; Rocca, C.; Giglio, A.; Angelone, T. Cardiometabolism as an Interlocking Puzzle between the Healthy and Diseased Heart: New Frontiers in Therapeutic Applications. J. Clin. Med. 2021, 10, 721. [Google Scholar] [CrossRef]
- Sletten, A.C.; Peterson, L.R.; Schaffer, J.E. Manifestations and mechanisms of myocardial lipotoxicity in obesity. J. Intern. Med. 2018, 284, 478–491. [Google Scholar] [CrossRef] [Green Version]
- Joseph, L.C.; Barca, E.; Subramanyam, P.; Komrowski, M.; Pajvani, U.; Colecraft, H.M.; Hirano, M.; Morrow, J.P. Inhibition of NAPDH Oxidase 2 (NOX2) Prevents Oxidative Stress and Mitochondrial Abnormalities Caused by Saturated Fat in Cardiomyocytes. PLoS ONE 2016, 11, e0145750. [Google Scholar] [CrossRef]
- Ly, L.D.; Xu, S.; Choi, S.K.; Ha, C.M.; Thoudam, T.; Cha, S.K.; Wiederkehr, A.; Wollheim, C.B.; Lee, I.K.; Park, K.S. Oxidative stress and calcium dysregulation by palmitate in type 2 diabetes. Exp. Mol. Med. 2017, 49, e291. [Google Scholar] [CrossRef] [Green Version]
- Park, M.; Sabetski, A.; Kwan Chan, Y.; Turdi, S.; Sweeney, G. Palmitate induces ER stress and autophagy in H9c2 cells: Implications for apoptosis and adiponectin resistance. J. Cell Physiol. 2015, 230, 630–639. [Google Scholar] [CrossRef]
- Kim, J.K.; Fillmore, J.J.; Sunshine, M.J.; Albrecht, B.; Higashimori, T.; Kim, D.W.; Liu, Z.X.; Soos, T.J.; Cline, G.W.; O’Brien, W.R.; et al. PKC-theta knockout mice are protected from fat-induced insulin resistance. J. Clin. Investig. 2004, 114, 823–827. [Google Scholar] [CrossRef] [Green Version]
- Drosatos, K.; Bharadwaj, K.G.; Lymperopoulos, A.; Ikeda, S.; Khan, R.; Hu, Y.; Agarwal, R.; Yu, S.; Jiang, H.; Steinberg, S.F.; et al. Cardiomyocyte lipids impair β-adrenergic receptor function via PKC activation. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E489–E499. [Google Scholar] [CrossRef] [Green Version]
- Listenberger, L.L.; Han, X.; Lewis, S.E.; Cases, S.; Farese, R.V., Jr.; Ory, D.S.; Schaffer, J.E. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc. Natl. Acad. Sci. USA 2003, 100, 3077–3082. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Qian, Y.; Fang, Q.; Zhong, P.; Li, W.; Wang, L.; Fu, W.; Zhang, Y.; Xu, Z.; Li, X.; et al. Saturated palmitic acid induces myocardial inflammatory injuries through direct binding to TLR4 accessory protein MD2. Nat. Commun. 2017, 8, 13997. [Google Scholar] [CrossRef] [Green Version]
- Yarmohammadi, F.; Hayes, A.W.; Karimi, G. Natural and chemical compounds as protective agents against cardiac lipotoxicity. Biomed. Pharmacother. 2022, 145, 112413. [Google Scholar] [CrossRef]
- Alam, W.; Rocca, C.; Khan, H.; Hussain, Y.; Aschner, M.; De Bartolo, A.; Amodio, N.; Angelone, T.; Cheang, W.S. Current Status and Future Perspectives on Therapeutic Potential of Apigenin: Focus on Metabolic-Syndrome-Dependent Organ Dysfunction. Antioxidants 2021, 10, 1643. [Google Scholar] [CrossRef]
- Casacchia, T.; Occhiuzzi, M.A.; Grande, F.; Rizzuti, B.; Granieri, M.C.; Rocca, C.; Gattuso, A.; Garofalo, A.; Angelone, T.; Statti, G. A pilot study on the nutraceutical properties of the Citrus hybrid Tacle® as a dietary source of polyphenols for supplementation in metabolic disorders. J. Funct. Foods 2019, 52, 370–381. [Google Scholar] [CrossRef]
- Casacchia, T.; Scavello, F.; Rocca, C.; Granieri, M.C.; Beretta, G.; Amelio, D.; Gelmini, F.; Spena, A.; Mazza, R.; Toma, C.C.; et al. Leopoldia comosa prevents metabolic disorders in rats with high-fat diet-induced obesity. Eur. J. Nutr. 2019, 58, 965–979. [Google Scholar] [CrossRef]
- Nettore, I.C.; Rocca, C.; Mancino, G.; Albano, L.; Amelio, D.; Grande, F.; Puoci, F.; Pasqua, T.; Desiderio, S.; Mazza, R.; et al. Quercetin and its derivative Q2 modulate chromatin dynamics in adipogenesis and Q2 prevents obesity and metabolic disorders in rats. J. Nutr. Biochem. 2019, 69, 151–162. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Wang, T.; Long, M.; Li, P. Quercetin: Its Main Pharmacological Activity and Potential Application in Clinical Medicine. Oxid. Med. Cell Longev. 2020, 2020, 8825387. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.H. Quercetin reduces high-fat diet-induced fat accumulation in the liver by regulating lipid metabolism genes. Phytother. Res. 2013, 27, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Panchal, S.K.; Poudyal, H.; Brown, L. Quercetin Ameliorates Cardiovascular, Hepatic, and Metabolic Changes in Diet-Induced Metabolic Syndrome in Rats. J. Nutr. 2012, 142, 1026–1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papakyriakopoulou, P.; Velidakis, N.; Khattab, E.; Valsami, G.; Korakianitis, I.; Kadoglou, N.P. Potential Pharmaceutical Applications of Quercetin in Cardiovascular Diseases. Pharmaceuticals 2022, 15, 1019. [Google Scholar] [CrossRef]
- Grande, F.; Parisi, O.I.; Mordocco, R.A.; Rocca, C.; Puoci, F.; Scrivano, L.; Quintieri, A.M.; Cantafio, P.; Ferla, S.; Brancale, A.; et al. Quercetin derivatives as novel antihypertensive agents: Synthesis and physiological characterization. Eur. J. Pharm. Sci. 2016, 82, 161–170. [Google Scholar] [CrossRef]
- Iacopetta, D.; Grande, F.; Caruso, A.; Mordocco, R.A.; Plutino, M.R.; Scrivano, L.; Ceramella, J.; Muià, N.; Saturnino, C.; Puoci, F.; et al. New insights for the use of quercetin analogs in cancer treatment. Future Med. Chem. 2017, 9, 2011–2028. [Google Scholar] [CrossRef]
- Rocca, C.; De Bartolo, A.; Grande, F.; Rizzuti, B.; Pasqua, T.; Giordano, F.; Granieri, M.C.; Occhiuzzi, M.A.; Garofalo, A.; Amodio, N.; et al. Cateslytin abrogates lipopolysaccharide-induced cardiomyocyte injury by reducing inflammation and oxidative stress through toll like receptor 4 interaction. Int. Immunopharmacol. 2021, 94, 107487. [Google Scholar] [CrossRef]
- Rocca, C.; Grande, F.; Granieri, M.C.; Colombo, B.; De Bartolo, A.; Giordano, F.; Rago, V.; Amodio, N.; Tota, B.; Cerra, M.C.; et al. The chromogranin A1-373 fragment reveals how a single change in the protein sequence exerts strong cardioregulatory effects by engaging neuropilin-1. Acta Physiol. (Oxf). 2021, 231, e13570. [Google Scholar] [CrossRef]
- Grande, F.; De Bartolo, A.; Occhiuzzi, M.A.; Caruso, A.; Rocca, C.; Pasqua, T.; Carocci, A.; Rago, V.; Angelone, T.; Sinicropi, M.S. Carbazole and Simplified Derivatives: Novel Tools toward β-Adrenergic Receptors Targeting. Appl. Sci. 2021, 11, 5486. [Google Scholar] [CrossRef]
- Rocca, C.; De Bartolo, A.; Granieri, M.C.; Rago, V.; Amelio, D.; Falbo, F.; Malivindi, R.; Mazza, R.; Cerra, M.C.; Boukhzar, L.; et al. The Antioxidant Selenoprotein T Mimetic, PSELT, Induces Preconditioning-like Myocardial Protection by Relieving Endoplasmic-Reticulum Stress. Antioxidants 2022, 11, 571. [Google Scholar] [CrossRef]
- Dong, Q.; Chen, L.; Lu, Q.; Sharma, S.; Li, L.; Morimoto, S.; Wang, G. Quercetin attenuates doxorubicin cardiotoxicity by modulating Bmi-1 expression. Br. J. Pharmacol. 2014, 171, 4440–4454. [Google Scholar] [CrossRef] [Green Version]
- McQueen, M.J. Optimal Assay of LDH and α-HBD at 37 °C. Ann. Clin. Biochem. 1972, 9, 21–25. [Google Scholar] [CrossRef] [Green Version]
- Rocca, C.; Scavello, F.; Colombo, B.; Gasparri, A.M.; Dallatomasina, A.; Granieri, M.C.; Amelio, D.; Pasqua, T.; Cerra, M.C.; Tota, B.; et al. Physiological levels of chromogranin A prevent doxorubicin-induced cardiotoxicity without impairing its anticancer activity. FASEB J. 2019, 33, 7734–7747. [Google Scholar] [CrossRef]
- Ivan, A.; Herman, H.; Balta, C.; Hadaruga, D.I.; Mihali, C.V.; Ardelean, A.; Hermenean, A. Berberis vulgaris extract/β-cyclodextrin complex increases protection of hepatic cells via suppression of apoptosis and lipogenesis pathways. Exp. Ther. Med. 2017, 13, 2143–2150. [Google Scholar] [CrossRef] [Green Version]
- Shen, C.J.; Kong, B.; Shuai, W.; Liu, Y.; Wang, G.J.; Xu, M.; Zhao, J.J.; Fang, J.; Fu, H.; Jiang, X.B.; et al. Myeloid differentiation protein 1 protected myocardial function against high-fat stimulation induced pathological remodelling. J. Cell Mol. Med. 2019, 23, 5303–5316. [Google Scholar] [CrossRef] [Green Version]
- Nasci, V.L.; Chuppa, S.; Griswold, L.; Goodreau, K.A.; Dash, R.K.; Kriegel, A.J. miR-21-5p regulates mitochondrial respiration and lipid content in H9C2 cells. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H710–H721. [Google Scholar] [CrossRef]
- Assimakopoulos, S.F.; Vagianos, C.E.; Zervoudakis, G.; Filos, K.S.; Georgiou, C.; Nikolopoulou, V.; Scopa, C.D. Gut regulatory peptides bombesin and neurotensin reduce hepatic oxidative stress and histological alterations in bile duct ligated rats. Regul. Pept. 2004, 120, 185–193. [Google Scholar] [CrossRef]
- Preetha Rani, M.R.; Anupama, N.; Sreelekshmi, M.; Raghu, K.G. Chlorogenic acid attenuates glucotoxicity in H9c2 cells via inhibition of glycation and PKC α upregulation and safeguarding innate antioxidant status. Biomed. Pharmacother. 2018, 100, 467–477. [Google Scholar] [CrossRef]
- Reznick, A.Z.; Packer, L. Oxidative damage to proteins: Spectrophotometric method for carbonyl assay. Methods Enzymol. 1994, 233, 357–363. [Google Scholar]
- Pasqua, T.; Rocca, C.; Lupi, F.R.; Baldino, N.; Amelio, D.; Parisi, O.I.; Granieri, M.C.; De Bartolo, A.; Lauria, A.; Dattilo, M.; et al. Cardiac and Metabolic Impact of Functional Foods with Antioxidant Properties Based on Whey Derived Proteins Enriched with Hemp Seed Oil. Antioxidants 2020, 9, 1066. [Google Scholar] [CrossRef]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Park, E.J.; Lee, A.Y.; Park, S.; Kim, J.H.; Cho, M.H. Multiple pathways are involved in palmitic acid-induced toxicity. Food Chem. Toxicol. 2014, 67, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Severson, D.L. Diabetic cardiomyopathy: Recent evidence from mouse models of type 1 and type 2 diabetes. Can. J. Physiol. Pharmacol. 2004, 82, 813–823. [Google Scholar] [CrossRef]
- van den Brom, C.E.; Huisman, M.C.; Vlasblom, R.; Boontje, N.M.; Duijst, S.; Lubberink, M.; Molthoff, C.F.; Lammertsma, A.A.; van der Velden, J.; Boer, C.; et al. Altered myocardial substrate metabolism is associated with myocardial dysfunction in early diabetic cardiomyopathy in rats: Studies using positron emission tomography. Cardiovasc. Diabetol. 2009, 8, 39. [Google Scholar] [CrossRef] [Green Version]
- Grundy, S.M.; Benjamin, I.J.; Burke, G.L.; Chait, A.; Eckel, R.H.; Howard, B.V.; Mitch, W.; Smith, S.C., Jr.; Sowers, J.R. Diabetes and cardiovascular disease: A statement for healthcare professionals from the American Heart Association. Circulation 1999, 100, 1134–1146. [Google Scholar] [CrossRef] [Green Version]
- Nichols, G.A.; Gullion, C.M.; Koro, C.E.; Ephross, S.A.; Brown, J.B. The incidence of congestive heart failure in type 2 diabetes: An update. Diabetes Care 2004, 27, 1879–1884. [Google Scholar] [CrossRef] [Green Version]
- Wende, A.R.; Abel, E.D. Lipotoxicity in the heart. Biochim. Biophys. Acta 2010, 1801, 311–319. [Google Scholar] [CrossRef]
- Kuwabara, Y.; Horie, T.; Baba, O.; Watanabe, S.; Nishiga, M.; Usami, S.; Izuhara, M.; Nakao, T.; Nishino, T.; Otsu, K.; et al. MicroRNA-451 exacerbates lipotoxicity in cardiac myocytes and high-fat diet-induced cardiac hypertrophy in mice through suppression of the LKB1/AMPK pathway. Circ. Res. 2015, 116, 279–288. [Google Scholar] [CrossRef] [Green Version]
- Urso, C.J.; Zhou, H. Role of CD36 in Palmitic Acid Lipotoxicity in Neuro-2a Neuroblastoma Cells. Biomolecules 2021, 11, 1567. [Google Scholar] [CrossRef]
- Hescheler, J.; Meyer, R.; Plant, S.; Krautwurst, D.; Rosenthal, W.; Schultz, G. Morphological, biochemical, and electrophysiological characterization of a clonal cell (H9c2) line from rat heart. Circ. Res. 1991, 69, 1476–1486. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Chen, Y.; Yan, Z.; Xu, T.T.; Wu, X.; Pi, A.; Liu, Q.; Chai, H.; Li, S.; Dou, X. Inhibition of TLR4/MAPKs Pathway Contributes to the Protection of Salvianolic Acid Against Lipotoxicity-Induced Myocardial Damage in Cardiomyocytes and Obese Mice. Front. Pharmacol. 2021, 12, 627123. [Google Scholar] [CrossRef]
- Kong, J.Y.; Rabkin, S.W. Palmitate-induced apoptosis in cardiomyocytes is mediated through alterations in mitochondria: Prevention by cyclosporin A. Biochim. Biophys. Acta 2000, 1485, 45–55. [Google Scholar] [CrossRef]
- Leroy, C.; Tricot, S.; Lacour, B.; Grynberg, A. Protective effect of eicosapentaenoic acid on palmitate-induced apoptosis in neonatal cardiomyocytes. Biochim. Biophys. Acta 2008, 1781, 685–693. [Google Scholar] [CrossRef]
- Di Majo, D.; Sardo, P.; Giglia, G.; Di Liberto, V.; Zummo, F.P.; Zizzo, M.G.; Caldara, G.F.; Rappa, F.; Intili, G.; van Dijk, R.M.; et al. Correlation of Metabolic Syndrome with Redox Homeostasis Biomarkers: Evidence from High-Fat Diet Model in Wistar Rats. Antioxidants 2023, 12, 89. [Google Scholar] [CrossRef]
- Chen, X.; Peng, X.; Luo, Y.; You, J.; Yin, D.; Xu, Q.; He, H.; He, M. Quercetin protects cardiomyocytes against doxorubicin-induced toxicity by suppressing oxidative stress and improving mitochondrial function via 14-3-3γ. Toxicol. Mech. Methods 2019, 29, 344–354. [Google Scholar] [CrossRef]
- Abushouk, A.I.; Ismail, A.; Salem, A.M.A.; Afifi, A.M.; Abdel-Daim, M.M. Cardioprotective mechanisms of phytochemicals against doxorubicin-induced cardiotoxicity. Biomed. Pharmacother. 2017, 90, 935–946. [Google Scholar] [CrossRef]
- Wang, S.Q.; Zhu, X.F.; Wang, X.N.; Shen, T.; Xiang, F.; Lou, H.X. Flavonoids from Malus hupehensis and their cardioprotective effects against doxorubicin-induced toxicity in H9c2 cells. Phytochemistry 2013, 87, 119–125. [Google Scholar] [CrossRef]
- Ojha, S.; Al Taee, H.; Goyal, S.; Mahajan, U.B.; Patil, C.R.; Arya, D.S.; Rajesh, M. Cardioprotective Potentials of Plant-Derived Small Molecules against Doxorubicin Associated Cardiotoxicity. Oxid. Med. Cell Longev. 2016, 2016, 5724973. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.W.; Chou, H.C.; Lin, S.T.; Chen, Y.H.; Chang, Y.J.; Chen, L.; Chan, H.L. Cardioprotective Effects of Quercetin in Cardiomyocyte under Ischemia/Reperfusion Injury. Evid Based Complement Alternat Med. 2013, 2013, 364519. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Zhang, H.; Chen, K.; Jin, M.; Vu, S.H.; Jung, S.; He, N.; Zheng, Z.; Lee, M.S. Application of chitosan/alginate nanoparticle in oral drug delivery systems: Prospects and challenges. Drug Deliv. 2022, 29, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Zhang, T.; Meng, Q.; Wang, S.; Yan, P.; Wang, X.; Luo, D.; Zhou, X.; Ji, R. Quercetin Improves Cardiomyocyte Vulnerability to Hypoxia by Regulating SIRT1/TMBIM6-Related Mitophagy and Endoplasmic Reticulum Stress. Oxid. Med. Cell Longev. 2021, 2021, 5529913. [Google Scholar] [CrossRef]
- Jain, A.K.; Mehra, N.K.; Swarnakar, N.K. Role of Antioxidants for the Treatment of Cardiovascular Diseases: Challenges and Opportunities. Curr. Pharm. Des. 2015, 21, 4441–4455. [Google Scholar] [CrossRef] [PubMed]
- Chimento, A.; Sala, M.; Gomez-Monterrey, I.M.; Musella, S.; Bertamino, A.; Caruso, A.; Sinicropi, M.S.; Sirianni, R.; Puoci, F.; Parisi, O.I.; et al. Biological activity of 3-chloro-azetidin-2-one derivatives having interesting antiproliferative activity on human breast cancer cell lines. Bioorg. Med. Chem. Lett. 2013, 23, 6401–6405. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Park, K.S.; Lee, C.; Park, H.R.; Choo, H.; Chong, Y. Enhanced stability and intracellular accumulation of quercetin by protection of the chemically or metabolically susceptible hydroxyl groups with a pivaloxymethyl (POM) promoiety. J. Med. Chem. 2010, 53, 8597–8607. [Google Scholar] [CrossRef]
- Kandemir, K.; Tomas, M.; McClements, D.J.; Capanoglu, E. Recent advances on the improvement of quercetin bioavailability. Trends Food Sci. Technol. 2022, 119, 192–200. [Google Scholar]
- Wei, C.D.; Li, Y.; Zheng, H.Y.; Tong, Y.Q.; Dai, W. Palmitate induces H9c2 cell apoptosis by increasing reactive oxygen species generation and activation of the ERK1/2 signaling pathway. Mol. Med. Rep. 2013, 7, 855–861. [Google Scholar] [CrossRef] [Green Version]
- Wei, C.D.; Li, Y.; Zheng, H.Y.; Sun, K.S.; Tong, Y.Q.; Dai, W.; Wu, W.; Bao, A.Y. Globular adiponectin protects H9c2 cells from palmitate-induced apoptosis via Akt and ERK1/2 signaling pathways. Lipids Health Dis. 2012, 11, 135. [Google Scholar] [CrossRef] [Green Version]
- Zou, L.; Li, X.; Wu, N.; Jia, P.; Liu, C.; Jia, D. Palmitate induces myocardial lipotoxic injury via the endoplasmic reticulum stress-mediated apoptosis pathway. Mol. Med. Rep. 2017, 16, 6934–6939. [Google Scholar] [CrossRef] [Green Version]
- Cai, L.; Wang, Y.; Zhou, G.; Chen, T.; Song, Y.; Li, X.; Kang, Y.J. Attenuation by metallothionein of early cardiac cell death via suppression of mitochondrial oxidative stress results in a prevention of diabetic cardiomyopathy. J. Am. Coll. Cardiol. 2006, 48, 1688–1697. [Google Scholar] [CrossRef] [Green Version]
- Cai, L. Suppression of nitrative damage by metallothionein in diabetic heart contributes to the prevention of cardiomyopathy. Free Radic. Biol. Med. 2006, 41, 851–861. [Google Scholar] [CrossRef]
- Li, Y.J.; Wang, P.H.; Chen, C.; Zou, M.H.; Wang, D.W. Improvement of mechanical heart function by trimetazidine in db/db mice. Acta Pharmacol. Sin. 2010, 31, 560–569. [Google Scholar] [CrossRef] [Green Version]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef]
- Rocca, C.; Pasqua, T.; Boukhzar, L.; Anouar, Y.; Angelone, T. Progress in the emerging role of selenoproteins in cardiovascular disease: Focus on endoplasmic reticulum-resident selenoproteins. Cell Mol. Life Sci. 2019, 76, 3969–3985. [Google Scholar] [CrossRef]
- Wu, K.M.; Hsu, Y.M.; Ying, M.C.; Tsai, F.J.; Tsai, C.H.; Chung, J.G.; Yang, J.S.; Tang, C.H.; Cheng, L.Y.; Su, P.H.; et al. High-density lipoprotein ameliorates palmitic acid-induced lipotoxicity and oxidative dysfunction in H9c2 cardiomyoblast cells via ROS suppression. Nutr. Metab. 2019, 16, 36. [Google Scholar] [CrossRef] [Green Version]
- Qian, P.; Tian, H.; Wang, Y.; Lu, W.; Li, Y.; Ma, T.; Gao, X.; Yao, W. A novel oral glucagon-like peptide 1 receptor agonist protects against diabetic cardiomyopathy via alleviating cardiac lipotoxicity induced mitochondria dysfunction. Biochem. Pharmacol. 2020, 182, 114209. [Google Scholar] [CrossRef]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, 71. [Google Scholar] [CrossRef] [Green Version]
- Roslan, J.; Giribabu, N.; Karim, K.; Salleh, N. Quercetin ameliorates oxidative stress, inflammation and apoptosis in the heart of streptozotocin-nicotinamide-induced adult male diabetic rats. Biomed. Pharmacother. 2017, 86, 570–582. [Google Scholar] [CrossRef]
- Yu, S.; Kim, S.R.; Jiang, K.; Ogrodnik, M.; Zhu, X.Y.; Ferguson, C.M.; Tchkonia, T.; Lerman, A.; Kirkland, J.L.; Lerman, L.O. Quercetin Reverses Cardiac Systolic Dysfunction in Mice Fed with a High-Fat Diet: Role of Angiogenesis. Oxid. Med. Cell Longev. 2021, 2021, 8875729. [Google Scholar] [CrossRef]
- Doroshow, J.H.; Locker, G.Y.; Myers, C.E. Enzymatic defenses of the mouse heart against reactive oxygen metabolites: Alterations produced by doxorubicin. J. Clin. Investig. 1980, 65, 128–135. [Google Scholar] [CrossRef] [Green Version]
- Rocca, C.; Pasqua, T.; Cerra, M.C.; Angelone, T. Cardiac Damage in Anthracyclines Therapy: Focus on Oxidative Stress and Inflammation. Antioxid. Redox Signal. 2020, 32, 1081–1097. [Google Scholar] [CrossRef] [PubMed]
- Nishi, H.; Higashihara, T.; Inagi, R. Lipotoxicity in Kidney, Heart, and Skeletal Muscle Dysfunction. Nutrients 2019, 11, 1664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warbrick, I.; Rabkin, S.W. Hypoxia-inducible factor 1-alpha (HIF-1α) as a factor mediating the relationship between obesity and heart failure with preserved ejection fraction. Obes. Rev. 2019, 20, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Fillmore, N.; Mori, J.; Lopaschuk, G.D. Mitochondrial fatty acid oxidation alterations in heart failure, ischaemic heart disease and diabetic cardiomyopathy. Br. J. Pharmacol. 2014, 171, 2080–2090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mangali, S.; Bhat, A.; Udumula, M.P.; Dhar, I.; Sriram, D.; Dhar, A. Inhibition of protein kinase R protects against palmitic acid-induced inflammation, oxidative stress, and apoptosis through the JNK/NF-kB/NLRP3 pathway in cultured H9C2 cardiomyocytes. J. Cell Biochem. 2019, 120, 3651–3663. [Google Scholar] [CrossRef]
- Zhang, K.; Kaufman, R.J. From endoplasmic-reticulum stress to the inflammatory response. Nature 2008, 454, 455–462. [Google Scholar] [CrossRef] [Green Version]
- Glass, C.K.; Olefsky, J.M. Inflammation and lipid signaling in the etiology of insulin resistance. Cell Metab. 2012, 15, 635–645. [Google Scholar] [CrossRef] [Green Version]
- Maloney, E.; Sweet, I.R.; Hockenbery, D.M.; Pham, M.; Rizzo, N.O.; Tateya, S.; Handa, P.; Schwartz, M.W.; Kim, F. Activation of NF-kappaB by palmitate in endothelial cells: A key role for NADPH oxidase-derived superoxide in response to TLR4 activation. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1370–1375. [Google Scholar] [CrossRef] [Green Version]
- Niebauer, J. Inflammatory mediators in heart failure. Int. J. Cardiol. 2000, 72, 209–213. [Google Scholar] [CrossRef]
- Shan, K.; Kurrelmeyer, K.; Seta, Y.; Wang, F.; Dibbs, Z.; Deswal, A.; Lee-Jackson, D.; Mann, D.L. The role of cytokines in disease progression in heart failure. Curr. Opin. Cardiol. 1997, 12, 218–223. [Google Scholar] [CrossRef]
- Sharma, R.; Coats, A.J.; Anker, S.D. The role of inflammatory mediators in chronic heart failure: Cytokines, nitric oxide, and endothelin-1. Int. J. Cardiol. 2000, 72, 175–186. [Google Scholar] [CrossRef]
- Forney, L.A.; Lenard, N.R.; Stewart, L.K.; Henagan, T.M. Dietary Quercetin Attenuates Adipose Tissue Expansion and Inflammation and Alters Adipocyte Morphology in a Tissue-Specific Manner. Int. J. Mol. Sci. 2018, 19, 895. [Google Scholar] [CrossRef] [Green Version]
- Sato, S.; Mukai, Y. Modulation of Chronic Inflammation by Quercetin: The Beneficial Effects on Obesity. J. Inflamm. Res. 2020, 13, 421–431. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Granieri, M.C.; Rocca, C.; De Bartolo, A.; Nettore, I.C.; Rago, V.; Romeo, N.; Ceramella, J.; Mariconda, A.; Macchia, P.E.; Ungaro, P.; et al. Quercetin and Its Derivative Counteract Palmitate-Dependent Lipotoxicity by Inhibiting Oxidative Stress and Inflammation in Cardiomyocytes. Int. J. Environ. Res. Public Health 2023, 20, 3492. https://doi.org/10.3390/ijerph20043492
Granieri MC, Rocca C, De Bartolo A, Nettore IC, Rago V, Romeo N, Ceramella J, Mariconda A, Macchia PE, Ungaro P, et al. Quercetin and Its Derivative Counteract Palmitate-Dependent Lipotoxicity by Inhibiting Oxidative Stress and Inflammation in Cardiomyocytes. International Journal of Environmental Research and Public Health. 2023; 20(4):3492. https://doi.org/10.3390/ijerph20043492
Chicago/Turabian StyleGranieri, Maria Concetta, Carmine Rocca, Anna De Bartolo, Immacolata Cristina Nettore, Vittoria Rago, Naomi Romeo, Jessica Ceramella, Annaluisa Mariconda, Paolo Emidio Macchia, Paola Ungaro, and et al. 2023. "Quercetin and Its Derivative Counteract Palmitate-Dependent Lipotoxicity by Inhibiting Oxidative Stress and Inflammation in Cardiomyocytes" International Journal of Environmental Research and Public Health 20, no. 4: 3492. https://doi.org/10.3390/ijerph20043492
APA StyleGranieri, M. C., Rocca, C., De Bartolo, A., Nettore, I. C., Rago, V., Romeo, N., Ceramella, J., Mariconda, A., Macchia, P. E., Ungaro, P., Sinicropi, M. S., & Angelone, T. (2023). Quercetin and Its Derivative Counteract Palmitate-Dependent Lipotoxicity by Inhibiting Oxidative Stress and Inflammation in Cardiomyocytes. International Journal of Environmental Research and Public Health, 20(4), 3492. https://doi.org/10.3390/ijerph20043492