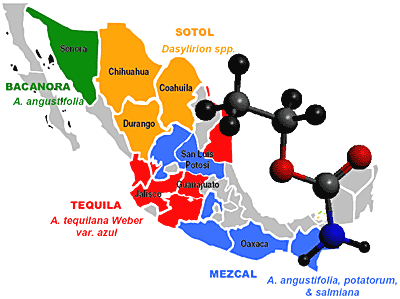
Ethyl Carbamate in Alcoholic Beverages from Mexico (Tequila, Mezcal, Bacanora, Sotol) and Guatemala (Cuxa): Market Survey and Risk Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Analysis of Ethyl Carbamate
2.3. Statistics
2.4. Approach for Risk Assessment
3. Results
4. Discussion
4.1. Mexico
4.2. Guatemala
5. Conclusions
Acknowledgments
References
- Dennis, MJ; Howarth, N; Key, PE; Pointer, M; Massey, RC. Investigation of ethyl carbamate levels in some fermented foods and alcoholic beverages. Food Addit. Contam 1989, 6, 383–389. [Google Scholar]
- Battaglia, R; Conacher, HBS; Page, BD. Ethyl carbamate (urethane) in alcoholic beverages and foods: a review. Food Addit. Contam 1990, 7, 477–496. [Google Scholar]
- Schlatter, J; Lutz, WK. The carcinogenic potential of ethyl carbamate (urethane): risk assessment at human dietary exposure levels. Food Chem. Toxicol 1990, 28, 205–211. [Google Scholar]
- Zimmerli, B; Schlatter, J. Ethyl carbamate: analytical methodology, occurrence, formation, biological activity and risk assessment. Mutat. Res 1991, 259, 325–350. [Google Scholar]
- Sen, NP; Seaman, SW; Boyle, M; Weber, D. Methyl carbamate and ethyl carbamate in alcoholic beverages and other fermented foods. Food Chem 1993, 48, 359–366. [Google Scholar]
- Lachenmeier, DW; Schehl, B; Kuballa, T; Frank, W; Senn, T. Retrospective trends and current status of ethyl carbamate in German stone-fruit spirits. Food Addit. Contam 2005, 22, 397–405. [Google Scholar]
- Conacher, HBS; Page, BD. Ethyl carbamate in alcoholic beverages: a canadian case history. Proceedings of Euro Food Tox II. European Society of Toxicology: Schwerzenbach, Switzerland, 1986; pp. 237–242. [Google Scholar]
- EFSA. Ethyl carbamate and hydrocyanic acid in food and beverages. EFSA J 2007, 551, 1–44.
- IARC Alcoholic Beverage Consumption and Ethyl Carbamate (Urethane), In Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France; in press.
- Vavasour, E; Renwick, AG; Engeli, B; Barlow, S; Castle, L; DiNovi, M; Slob, W; Schlatter, J; Bolger, M. Ethyl carbamate. WHO Food Additives Series 55. Safety evaluation of certain contaminants in food. In Prepared by the sixty-fourth meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA); WHO and FAO: Geneva, Switzerland, 2006; pp. 205–316. [Google Scholar]
- Stevens, G; Dias, RH; Thomas, KJ; Rivera, JA; Carvalho, N; Barquera, S; Hill, K; Ezzati, M. Characterizing the epidemiological transition in Mexico: national and subnational burden of diseases, injuries, and risk factors. PLoS Med 2008, 5, e125. [Google Scholar]
- Rehm, J; Room, R; Monteiro, M; Gmel, G; Graham, K; Rehn, N; Sempos, CT; Frick, U; Jernigan, D. Alcohol use. In Comparative Quantification of Health Risks. Global and Regional Burden of Disease Attributable to Selected Major Risk Factors. Volume 1; Ezzati, M, Lopez, AD, Rodgers, A, Murray, CJL, Eds.; World Health Organization: Geneva, 2004; pp. 959–1108. [Google Scholar]
- Room, R; Jernigan, D; Carlini-Marlatt, B; Gureje, O; Mäkelä, K; Marshall, M; Medina-Mora, ME; Monteiro, M; Parry, C; Partanen, J; Riley, L; Saxena, S. Alcohol in developing societies: a public health approach; Finnish Foundation for Alcohol Studies in collaboration with World Health Organization: Helsinki, Finland, 2002; p. 121. [Google Scholar]
- Narro-Robles, J; Gutiérrez-Avila, JH; Lopez-Cervantes, M; Borges, G; Rosovsky, H. Liver cirrhosis mortality in Mexico. II. Excess mortality and pulque consumption. Salud Publica Mex 1992, 34, 388–405. [Google Scholar]
- Narro-Robles, J; Gutiérrez-Avila, JH. Ecological correlation between consumption of alcoholic beverages and liver cirrhosis mortality in Mexico. Salud Publica Mex 1997, 39, 217–220. [Google Scholar]
- Cadranel, JF; Legendre, C; Desaint, B; Delamarre, N; Florent, C; Levy, VG. Liver disease from surreptitious administration of urethane. J. Clin. Gastroenterol 1993, 17, 52–56. [Google Scholar]
- Beland, FA; Benson, RW; Mellick, PW; Kovatch, RM; Roberts, DW; Fang, JL; Doerge, DR. Effect of ethanol on the tumorigenicity of urethane (ethyl carbamate) in B6C3F1 mice. Food. Chem. Toxicol 2005, 43, 1–19. [Google Scholar]
- Lachenmeier, DW; Sohnius, E-M; Attig, R; López, MG. Quantification of selected volatile constituents and anions in Mexican Agave spirits (Tequila, Mezcal, Sotol, Bacanora). J. Agric. Food Chem 2006, 54, 3911–3915. [Google Scholar]
- Kanteres, F; Lachenmeier, DW; Rehm, J. Alcohol in Mayan Guatemala: Consumption, distribution, production and composition of cuxa. Addiction, in press.
- Baumann, U; Zimmerli, B. Gas chromatographic determination of urethane (ethyl carbamate) in alcoholic beverages. Mitt. Geb. Lebensmittelunters. Hyg 1986, 77, 327–332. [Google Scholar]
- Mildau, G; Preuß, A; Frank, W; Heering, W. Ethyl carbamate (urethane) in alcoholic beverages: improved analysis and light-dependent formation. Deut. Lebensm. Rundsch 1987, 83, 69–74. [Google Scholar]
- Lachenmeier, DW; Frank, W; Kuballa, T. Application of tandem mass spectrometry combined with gas chromatography to the routine analysis of ethyl carbamate in stone-fruit spirits. Rapid Commun. Mass Spectrom 2005, 19, 108–112. [Google Scholar]
- Funch, F; Lisbjerg, S. Analysis of ethyl carbamate in alcoholic beverages. Z. Lebensmittelunters. Forsch 1988, 186, 29–32. [Google Scholar]
- EFSA. Opinion of the Scientific Committee on a request from EFSA related to a harmonised approach for risk assessment of substances which are both genotoxic and carcinogenic. EFSA J 2005, 282, 1–31.
- WHO. Global Information System on Alcohol and Health.
- Lachenmeier, DW; Richling, E; López, MG; Frank, W; Schreier, P. Multivariate analysis of FTIR and ion chromatographic data for the quality control of tequila. J. Agric. Food Chem 2005, 53, 2151–2157. [Google Scholar]
- Lachenmeier, DW. Evaluation of Tequila’s quality categories using sensory analysis. Deut. Lebensm. Rundsch 2006, 102, 400–404. [Google Scholar]
- FSANZ, Ethyl carbamate in Australian foods. Survey of sampling and analysis conducted 2007; Food Standards Australia New Zealand: Canberra, Australia, 2007.
- Hong, KP; Kang, YS; Jung, DC; Park, SR; Yoon, JH; Lee, SY; Ko, YS; Kim, SH; Ha, SD; Park, SK; Bae, DH. Exposure to ethyl carbamate by consumption of alcoholic beverages imported in Korea. Food Sci. Biotechnol 2007, 16, 975–980. [Google Scholar]
- Jones, DA. Why are so many food plants cyanogenic? Phytochemistry 1998, 47, 155–162. [Google Scholar]
- Sotelo, A; Lopez-Garcia, S; Basurto-Pena, F. Content of nutrient and antinutrient in edible flowers of wild plants in Mexico. Plant Foods Hum. Nutr 2007, 62, 133–138. [Google Scholar]
- Schehl, B; Senn, T; Lachenmeier, DW; Rodicio, R; Heinisch, JJ. Contribution of the fermenting yeast strain to ethyl carbamate generation in stone fruit spirits. Appl. Microbiol. Biotechnol 2007, 74, 843–850. [Google Scholar]
- Aresta, M; Boscolo, M; Franco, DW. Copper(II) catalysis in cyanide conversion into ethyl carbamate in spirits and relevant reactions. J. Agric. Food Chem 2001, 49, 2819–2824. [Google Scholar]
- Rehm, J; Klotsche, J; Patra, J. Comparative quantification of alcohol exposure as risk factor for global burden of disease. Int. J. Methods Psychiatr. Res 2007, 16, 66–76. [Google Scholar]
- Rehm, J; Patra, J; Baliunas, D; Popova, S; Roerecke, M; Taylor, B. Alcohol consumption and the global burden of disease 2002; WHO, Department of Mental Health and Substance Abuse, Management of Substance Abuse (Internal document for the meeting of the WHO Technical Advisory Group on Alcohol Epidemiology): Geneva, Switzerland, 2006. [Google Scholar]
- Bruno, SNF; Vaitsman, DS; Kunigami, CN; Brasil, MG. Influence of the distillation processes from Rio de Janeiro in the ethyl carbamate formation in Brazilian sugar cane spirits. Food Chem 2007, 104, 1345–1352. [Google Scholar]
- Faria, JB; Loyola, E; López, MG; Dufour, JP. Cachaça, pisco and tequila. In Fermented Beverage Production; Lea, AGH, Piggott, JR, Eds.; Kluwer Academic/Plenum Publishers: New York, USA, 2003; pp. 335–363. [Google Scholar]
- Acetaldehyde, IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol. 71, Re-Evaluation of Some Organic Chemicals, Hydrazine and Hydrogen Peroxide; International Agency for Research on Cancer: Lyon, France, 1999; pp. 319–335.
- Lachenmeier, DW; Sarsh, B; Rehm, J. The composition of alcohol products from markets in Lithuania and Hungary, and potential health consequences: A pilot study. Alcohol Alcohol 2009, 44, 93–102. [Google Scholar]
- Lachenmeier, DW; Kanteres, F; Rehm, J. Carcinogenicity of acetaldehyde in alcoholic beverages: risk assessment outside of ethanol metabolism. Addiction, in press.

| Country | Category | Numbers of samples in the range [mg/L]
| |||||
|---|---|---|---|---|---|---|---|
| < LOD (0.01) | < LOQ (0.04) | 0.04–0.1 | 0.1–0.2 | 0.2–0.3 | 0.3–0.4 | ||
| Mexico | Tequila | 9 | 12 | 18 | 7 | 0 | 0 |
| Mexico | Mezcal | 5 | 0 | 27 | 2 | 1 | 0 |
| Mexico | Sotol | 5 | 1 | 9 | 1 | 0 | 0 |
| Mexico | Bacanora | 5 | 0 | 7 | 0 | 0 | 1 |
| Guatemala | Clandestine Cuxa | 9 | 1 | 1 | 0 | 0 | 0 |
| Guatemala | Commercial Cuxa | 3 | 2 | 0 | 0 | 0 | 0 |
| Country | N | Ethyl carbamate [mg/L] a | |||||
|---|---|---|---|---|---|---|---|
| Mean | Median | 90th percentile | 95th percentile | 99th percentile | Maximum | ||
| Mexico, different states (agave spirits) | 110 | 0.05 | 0.04 | 0.10 | 0.14 | 0.22 | 0.39 |
| Guatemala, Nahualá (cuxa) | 16 | 0.01 | 0.00 | 0.03 | 0.04 | 0.06 | 0.06 |
| Scenarios for different ethyl carbamate concentrations | |||||
|---|---|---|---|---|---|
| Mean | Median | 90th percentile | 95th percentile | 99th percentile | |
| Exposure [ng/kg bw/day] | 6 | 5 | 11 | 16 | 25 |
| Margin of Exposure (MOE) | 52,560 | 65,700 | 26,280 | 18,771 | 11,945 |
| Intake scenarios | Exposure for different ethyl carbamate concentration scenarios in spirits [ng/kg bw/day] | |||||
|---|---|---|---|---|---|---|
| Drinks per day | Volume of spirit consumed [mL] | Mean | Median | 90th percentile | 95th percentile | 99th percentile |
| 1 | 25 | 21 | 17 | 42 | 58 | 92 |
| 2 | 50 | 42 | 33 | 83 | 117 | 183 |
| 3 | 75 | 63 | 50 | 125 | 175 | 275 |
| 4 | 100 | 83 | 67 | 167 | 233 | 367 |
| 5 | 125 | 104 | 83 | 208 | 292 | 458 |
| Intake scenarios | MOE for different exposure scenarios | |||||
|---|---|---|---|---|---|---|
| Drinks per day | Volume of spirit consumed [ml] | Mean | Median | 90th percentile | 95th percentile | 99th percentile |
| 1 | 25 | 14,400 | 18,000 | 7,200 | 5,143 | 3,273 |
| 2 | 50 | 7,200 | 9,000 | 3,600 | 2,571 | 1,636 |
| 3 | 75 | 4,800 | 6,000 | 2,400 | 1,714 | 1,091 |
| 4 | 100 | 3,600 | 4,500 | 1,800 | 1,286 | 818 |
| 5 | 125 | 2,880 | 3,600 | 1,440 | 1,029 | 655 |
Share and Cite
Lachenmeier, D.W.; Kanteres, F.; Kuballa, T.; López, M.G.; Rehm, J. Ethyl Carbamate in Alcoholic Beverages from Mexico (Tequila, Mezcal, Bacanora, Sotol) and Guatemala (Cuxa): Market Survey and Risk Assessment. Int. J. Environ. Res. Public Health 2009, 6, 349-360. https://doi.org/10.3390/ijerph6010349
Lachenmeier DW, Kanteres F, Kuballa T, López MG, Rehm J. Ethyl Carbamate in Alcoholic Beverages from Mexico (Tequila, Mezcal, Bacanora, Sotol) and Guatemala (Cuxa): Market Survey and Risk Assessment. International Journal of Environmental Research and Public Health. 2009; 6(1):349-360. https://doi.org/10.3390/ijerph6010349
Chicago/Turabian StyleLachenmeier, Dirk W., Fotis Kanteres, Thomas Kuballa, Mercedes G. López, and Jürgen Rehm. 2009. "Ethyl Carbamate in Alcoholic Beverages from Mexico (Tequila, Mezcal, Bacanora, Sotol) and Guatemala (Cuxa): Market Survey and Risk Assessment" International Journal of Environmental Research and Public Health 6, no. 1: 349-360. https://doi.org/10.3390/ijerph6010349
APA StyleLachenmeier, D. W., Kanteres, F., Kuballa, T., López, M. G., & Rehm, J. (2009). Ethyl Carbamate in Alcoholic Beverages from Mexico (Tequila, Mezcal, Bacanora, Sotol) and Guatemala (Cuxa): Market Survey and Risk Assessment. International Journal of Environmental Research and Public Health, 6(1), 349-360. https://doi.org/10.3390/ijerph6010349