Leachability of Arsenic and Heavy Metals from Mine Tailings of Abandoned Metal Mines
Abstract
:1. Introduction
2. Experimental
3. Results and Discussion
3.1. Physical and Chemical Characteristics of the Mine Tailings
3.2. Total Concentrations of As and Heavy Metals in the Mine Tailings
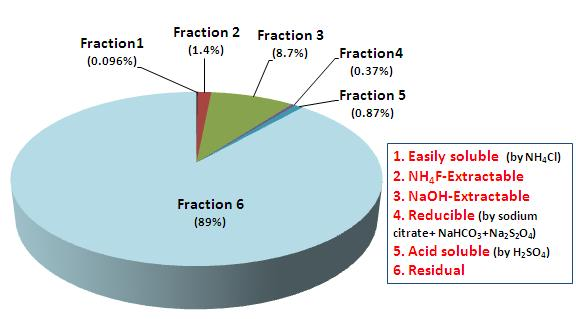
3.3. Chemical Distribution of As in the Mine Tailings by Sequential Extraction
3.4. Leaching of As and Heavy Metals from the Mine Tailings
4. Conclusions
Acknowledgments
References and Notes
- Roussel, C; Bril, H; Fernandez, A. Heavy metals in the environment. Arsenic speciation: involvement in evaluation of environmental impact caused by mine wastes. J. Environ. Qual 2000, 29, 182–188. [Google Scholar]
- Jang, M; Hwang, JS; Choi, SI; Park, JK. Remediation of arsenic-contaminated soils and washing effluents. Chemosphere 2005, 60, 344–354. [Google Scholar]
- Kim, M; Jung, Y. Vertical distribution and mobility of arsenic and heavy metals in and around mine tailings of an abandoned mine. J. Environ. Sci. Health Part A 2004, 39, 203–222. [Google Scholar]
- Blowes, D; Jambor, J; Hanton-Fong, C; Lortie, L; Gould, W. Geochemical, mineralogical and microbiological characterization of a sulphide-bearing carbonate-rich gold-mine tailings impoundment, Joutel, Quebec. Appl. Geochem 1998, 13, 687–705. [Google Scholar]
- McGregor, R; Blowes, D; Jambor, J; Robertson, W. The solid-phase controls on the mobility of heavy metals at the Copper Cliff tailings area, Sudbury, Ontario, Canada. J. Contam. Hydrol 1998, 33, 247–271. [Google Scholar]
- Lee, M; Paik, I; Kim, I; Kang, H; Lee, S. Remediation of heavy metal contaminated groundwater originated from abandoned mine using lime and calcium carbonate. J. Hazar. Mater 2007, 144, 208–214. [Google Scholar]
- Korean Ministry of Environment, Final report on soil environment control actions of abandoned metal mines; Korean Ministry of Environment: Gwachen, Gyeonggi-do, Korea, 2007.
- Cline, SR; Reed, BE. Lead removal from soils via bench-scale soil washing techniques. J. Environ. Engin 1995, 121, 700–705. [Google Scholar]
- Sirguey, C; de Souza e Silva, PT; Schwartz, C; Simonnot, M-O. Impact of chemical oxidation on soil quality. Chemosphere 2008, 72, 282–289. [Google Scholar]
- Basha, EA; Hashim, R; Mahmud, HB; Muntohar, AS. Stabilization of residual soil with rice husk ash and cement. Construc. Build. Mater 2005, 19, 448–453. [Google Scholar]
- Del Panno, MT; Morelli, IS; Engelen, B; Corti, LB. Effect of petrochemical sludge concentrations on microbial communities during soil bioremediation. FEMS Microbio. Ecolo 2005, 53, 305–316. [Google Scholar]
- Ahn, JW; Cho, HC; You, KS; Han, GC; Um, NI. Characteristics of carbonation reaction from municipal solid waste incinerator bottom ash as a function of water content and their effect on the stabilization of copper and lead. Mater. Sci. Forum 2007, 544, 533–536. [Google Scholar]
- Bertos, M Fernandez; Simons, SJR; Hills, CD; Carey, PJ. A review of accelerated carbonation technology in the treatment of cement‐based materials and sequestration of CO2. J. Hazar. Mater 2004, 112, 193–205. [Google Scholar]
- Melton, JS; Tarabadkar, K; Kevin, G. Accelerated Carbonation of Contaminated Soils for Beneficial Use Applications, Executive Summary of Project 34, Univ. of New Hampshire, Durham, NH, USA, 2008.
- Korean Ministry of Environment, Nov. 2004 (No. 2004-185), Waste Standard Test, Waste Management Act. Korean Ministry of Environment: Gwachen, Gyeonggi-do, Korea, 2004.
- EPA method 6010, Compilation of EPA’s Sampling and Analysis Methods, 2nd ed; Section A, CRC press, Lewis Publishers Inc: New York, NY, USA, 1998.
- Korean Ministry of Environment. July 2008 (No. 2008-115), Soil Environment Standard Test, Soil Environment Preservation Act.
- Wang, S; Mulligan, CN. Speciation and surface structure of inorganic arsenic in solid phases: A review. Environ. Intern 2008, 34, 867–879. [Google Scholar]
- Herreweghe, SV; Swennen, R; Vandecasteele, C; Cappuyns, V. Solid phase speciation of arsenic by sequential extraction in standard reference materials and industrially contaminated soil samples. Environ. Pollut 2003, 122, 323–342. [Google Scholar]
- .
- .
- Jung, M; Ahn, J; Chon, H. Environmental contamination and sequential extraction of trace elements from mine wastes around various metalliferrous mines in Korea. Geosystem Eng 2001, 4, 50–60. [Google Scholar]
- Kartinen, EO; Martin, CJ. An overview of arsenic removal processes. Desalination 1995, 103, 79–88. [Google Scholar]
- Johnston, SE; Barnard, W. Comparative effectiveness of fourteen solutions for extracting arsenic form four Western New York soils. Soil Sci. Soc. Am. J 1979, 43, 304–308. [Google Scholar]
- Drahota, P; Filippi, M. Secondary arsenic minerals in the environment: A review. Environ. Intern 2009, 35, 1243–1255. [Google Scholar]
- Kim, M; Ahn, K; Jung, Y. Distribution of inorganic species in mine tailings of abandoned mines from Korea. Chemosphere 2000, 49, 307–312. [Google Scholar]
- Ghosh, A; Mukiibi, M; Ela, W. TCLP underestimates leaching of arsenic from solid residuals under landfill conditions. Environ. Sci. Technol 2004, 38, 4677–4682. [Google Scholar]
- Ribit, I; Ptacek, C; Blowes, D; Jambor, J. The potential for metal release by reductive dissolution of weathered mine tailings. J. Contam. Hydrol 1995, 17, 239–273. [Google Scholar]
- Johnson, R; Blowes, D; Robertson, W; Jambor, J. The hydrogeochemistry of the Nickel Rim mine tailings impoundment, Sudbury, Ontario. J. Contam. Hydrol 2000, 41, 49–80. [Google Scholar]
- Holmstrom, H; Ljungberg, J; Ekstrom, M; Ohlander, B. Secondary copper enrichment in tailings at the Laver mine, northern Sweden. Environ. Geol 1999, 38, 327–342. [Google Scholar]




| EPA method 6010 | KST for soil | KST for waste | |
|---|---|---|---|
| Definition and purpose | To determine concentrations of trace elements, including metals, in groundwater, soils, sludges, sediments and other solid wastes | To determine if the soil is contaminated by either inorganic or organic environmental contaminants over the regulation level by Korean soil preservation act | To determine if a waste is hazardous over the regulation level by Korean waste management act and to determine concentrations of contaminants in a waste |
| Target samples | Sediment, sludge, and soil | Soil | Wastes including low content of organic matter and metallic oxide, hydroxide, and sulfide |
| Target elements | As, Ag, Al, Ba, Be, Ca, Co, Cr, Cu, Fe, K, Mg, Mn, Mo, Na, Ni, Pb, Se, Zn, Tl, V | Cd, Cu, Pb, As | As, Pb, Cd, Cu, Cr |
| Sample amount | 2 g | 10 g | Unspecified |
| Reagents | 1:1 HNO3, 30% H2O2, and concentrated HCl | 0.1 N HCl (for Cd, Cu, andPb) 1 N HCl (for As) | HNO3 and HCl (1 + 1) |
| Reaction | Heating (under boiling point) | Shaking (100 rpm for 1 hr at 30 °C) | Heating (under boiling point) |
| Total reaction time | About 3.5 hr | 1 hr | About 2 hr |
| Final solution volume (with water addition) | 100 mL | 50 mL | 100 mL |
| TCLP | KSLT | |
|---|---|---|
| Definition | An analysis method to determine the mobility of both organic and inorganic analytes present in liquid, solid, and multiphasic wastes | A analysis method to predict potential leaching level of environmental contaminants from industrial wastes after landfill |
| Purpose | To determine if a waste may meet the definition of EP (Extraction Procedure) Toxicity, that is, carrying a hazardous waste code (40CFR Part 261) under Resource Conservation and Recovery Act (RCRA) | To determine if a waste is specified over the regulation level of Korean waste management act or which landfill method is proper for a waste |
| Target solid materials | Materials are solid waste if they are abandoned by being: (1) Disposed of; or (2) Burned or incinerated; or (3) Accumulated, stored, or treated (but not recycled) before or in lieu of being abandoned by being disposed of, burned, or incinerated | Slag, dust, sand blast, waste refractory, incineration waste residue, solidified/stabilized waste, waste catalyst, waste absorbent/adsorbent, wastewater sludge, etc. |
| Sample treatment | Sieving into 9.5 mm | Sieving into 5.0–5.5 mm |
| Extraction device | Rotary extraction device (30 rpm) | Horizontal back-and-forth shaker (200 rpm) |
| Extraction time | 18 hr | 6 hr |
| pH of extractant | Fluid #1: pH 4.93 ± 0.05 Fluid #2: pH 2.88 ± 0.05 | pH 5.8–6.3 adding HCl to distilled water |
| Sample (g): Extractant (mL) | 1:20 | 1:10 |
| Separation of solid and liquid | 0.6–0.8 μm-membrane filter or centrifuge | 1 μm-membrane filter or centrifuge |
| Property | Unit | Value | |
|---|---|---|---|
| pH | 7.5 ± 0.14 | ||
| Water content | % | 9.6 ± 0.42 | |
| Loss on ignition (LOI) | % | 9.3 ± 0.33 | |
| Organic carbon content | % | 3.9 ± 0.15 | |
| Anions | F− | mg/L | 0.11 |
| Cl− | mg/L | 20 | |
| NO2− | mg/L | ND | |
| NO3− | mg/L | 1.9 | |
| Br− | mg/L | 0.71 | |
| PO42− | mg/L | 9.6 | |
| SO42− | mg/L | 224 | |
| Metal (mg/kg) | Standard method | Korean soil contamination criteria | |||||
|---|---|---|---|---|---|---|---|
| Acting | Warning | ||||||
| EPA method 6010 | KST for soil | KST for waste | A area | B area | A area | B area | |
| As | 67,336 ± 104 | 3,068 ± 22 | 66,155 ± 710 | 15 | 50 | 6 | 20 |
| Fe | 137,180 ± 756 | NE | NE | NE | NE | ||
| Cu | 764 ± 0.83 | 233 ± 1.67 | 745 ± 2 | 125 | 500 | 50 | 200 |
| Pb | 3,421 ± 20 | 875 ± 3.1 | 3,572 ± 51 | 300 | 1,000 | 100 | 400 |
| Mn | 24,256 ± 31 | NE | NE | NE | NE | ||
| Cr(VI) | 71.7 ± 0.67 | 65 ± 1.35 | 10 | 30 | 4 | 12 | |
| Cd | 54.4 ± 0.09 | 7.2 ± 0.5 | 56.3 ± 0.3 | 4 | 30 | 1.5 | 12 |
| Zn | 12,420 ± 4.0 | NE | NE | NE | NE | ||
| Step [19] | Extractant [19] | As chemical fraction [19] | Mineral (Formula) [25] | As concentration (Average) | Proportion (%) | |
|---|---|---|---|---|---|---|
| mg/L leachate | mg/kg tailings | |||||
| 1. Easily soluble | 1M NH4Cl (pH 7) | Neutral (non-ionic) As | Arsenolite (As2O3) Claudetite (As2O3) | 0.80 | 48 | 0.096 |
| 2. NH4F-extractable | 0.5M NH4F (pH 8) | As bound to Al | Mansfieldite (AlAsO4·2H2O) | 12 | 716 | 1.4 |
| 3. NaOH-extractable | 0.1M NaOH (pH 12) | As bound to Fe (non-occluded As) | Scorodite (FeAsO4·2H2O) Symplesite (Fe3(AsO4)2·8H2O) | 89 | 4,343 | 8.7 |
| 4. Reducible | 0.5M sodium citrate + 1M NaHCO3 + 0.5g Na2S2O4·2H2O | As bound to Fe oxide (Occluded As) | Kalfanite (Ca2Fe3O2(AsO4)·2H2O) | 3.1 | 183 | 0.37 |
| 5. Acid soluble | 0.25M H2SO4 (pH 1) | As bound to Ca | Rauenthalite (Ca3(AsO4)2·10H2O) Pharmacolite (Ca(HAsO4)·2H2O) | 7.3 | 435 | 0.87 |
| 6. Residual | HClconc + HNO3conc +HFconc | As bound to silicate and sulfide minerals | Arsenopyrite (FeAsS) | 440 | 44,023 | 89 |
| Metal | Leaching level (mg/L) | Criteria (mg/L) | ||
|---|---|---|---|---|
| TCLP (US EPA) | KSLT (Korea) | TCLP (US EPA) | KSLT (Korea) | |
| As | 0.43 | 0.24 | 5.0 | 1.5 |
| Pb | 0.15 | 0.10 | 5.0 | 3.0 |
| Cr(VI) | 0.42 | 0.36 | 5.0 | 1.5 |
| Cu | 0.29 | 0.08 | NE | 3.0 |
| Cd | 0.20 | 0.19 | 1.0 | 0.3 |
| Parameter | Unit | TCLP | KSLT | Sequential extraction | |
|---|---|---|---|---|---|
| Fraction 1 | Fraction 2 | ||||
| pH of extractant | pH | 5 | 6 | 7 | 8 |
| Extraction time | hr | 18 | 6 | 2 | 15 |
| Solid (g):liquid (mL) | 1:20 | 1:10 | 1:60 | 1:60 | |
| As concentration in leachant | mg/L | 0.43 | 0.24 | 0.80 | 12 |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lim, M.; Han, G.-C.; Ahn, J.-W.; You, K.-S.; Kim, H.-S. Leachability of Arsenic and Heavy Metals from Mine Tailings of Abandoned Metal Mines. Int. J. Environ. Res. Public Health 2009, 6, 2865-2879. https://doi.org/10.3390/ijerph6112865
Lim M, Han G-C, Ahn J-W, You K-S, Kim H-S. Leachability of Arsenic and Heavy Metals from Mine Tailings of Abandoned Metal Mines. International Journal of Environmental Research and Public Health. 2009; 6(11):2865-2879. https://doi.org/10.3390/ijerph6112865
Chicago/Turabian StyleLim, Mihee, Gi-Chun Han, Ji-Whan Ahn, Kwang-Suk You, and Hyung-Seok Kim. 2009. "Leachability of Arsenic and Heavy Metals from Mine Tailings of Abandoned Metal Mines" International Journal of Environmental Research and Public Health 6, no. 11: 2865-2879. https://doi.org/10.3390/ijerph6112865
APA StyleLim, M., Han, G. -C., Ahn, J. -W., You, K. -S., & Kim, H. -S. (2009). Leachability of Arsenic and Heavy Metals from Mine Tailings of Abandoned Metal Mines. International Journal of Environmental Research and Public Health, 6(11), 2865-2879. https://doi.org/10.3390/ijerph6112865