Smoking and the Risk of Upper Aero Digestive Tract Cancers for Men and Women in the Asia-Pacific Region
Abstract
:1. Introduction
1.1. Methods
2. Results and Discussion
2.1. Current Smoking and Risk of Mortality from UADTC
2.2. Current Drinking and Smoking and UADTC
2.3. Smoking and Risk of Mortality from UADTC in Former Smokers
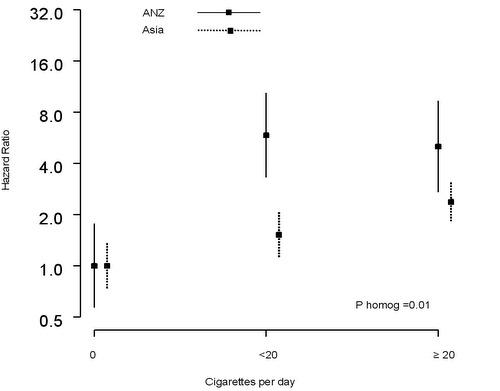
2.4. Dose-Response Relationship between Current Smoking and UADTC
2.5. Contribution of Smoking to Mortality from UADTC
2.6. Discussion
3. Conclusions
Acknowledgments
- Executive Committee: M. Woodward (Chair), R. Huxley, X. Fang, D.F. Gu, Y. Imai, T.H. Lam, W.H. Pan, A. Rodgers, I. Suh, H.C. Kim, H. Ueshima
- Funding/Support: This project has received grants from the National Health and Medical Research Council of Australia, the University of Sydney and an unrestricted educational grant from Pfizer Inc. Alireza Ansary-Moghaddam was funded by a scholarship from Zahedan University of Medical Sciences and Alexandra Martiniuk was funded by a postdoctoral fellowship from the Canadian Institutes of Health Research.
- Participating Studies and Principal Collaborators in APCSC: Aito Town: A. Okayama, H. Ueshima; H. Maegawa; Akabane: M. Nakamura, N. Aoki; Anzhen02: Z.S. Wu; Anzhen: C.H. Yao, Z.S. Wu; Australian Longitudinal Study of Aging: M. Luszcz; Australian National Heart Foundation: T.A. Welborn; Beijing Aging: Z. Tang; Beijing Steelworkers: L.S. Liu, J.X. Xie; Blood Donors’ Health: R. Norton, S. Ameratunga, S. MacMahon, G. Whitlock; Busselton: M.W. Knuiman; Canberra-Queanbeyan: H. Christensen; Capital Iron and Steel Company: X.G. Wu; CISCH: J. Zhou, X.H. Yu; Civil Service Workers: A. Tamakoshi; CVDFACTS: W.H. Pan; East Beijing: Z.L. Wu, L.Q. Chen, G.L. Shan; Electricity Generating Authority of Thailand: P. Sritara; Fangshan: D.F. Gu, X.F. Duan; Fletcher Challenge: S. MacMahon, R. Norton, G. Whitlock, R. Jackson; Guangzhou: Y.H. Li; Guangzhou Occupational: T.H. Lam, C.Q. Jiang; Hisayama: Y. Kiyohara, H. Arima, M. Iida; Hong Kong: J. Woo, S.C. Ho; Huashan: Z. Hong, M.S. Huang, B. Zhou (deceased); Kinmen: J.L. Fuh; Konan: H. Ueshima, Y. Kita, S.R. Choudhury; KMIC: I. Suh, S.H. Jee, I.S. Kim; Melbourne: G.G. Giles; Miyama: T. Hashimoto, K. Sakata; Newcastle: A. Dobson; Ohasama: Y. Imai, T. Ohkubo, A. Hozawa; Perth: K. Jamrozik, M. Hobbs, R. Broadhurst; Saitama: K. Nakachi; Seven Cities: X.H. Fang, S.C. Li, Q.D. Yang; Shanghai Factory Workers: Z.M. Chen; Shibata: H. Tanaka; Shigaraki Town: Y. Kita, A. Nozaki, H. Ueshima; Shirakawa: H. Horibe, Y. Matsutani, M. Kagaya; Singapore Heart: K. Hughes, J. Lee; Singapore NHS92: D. Heng, S.K. Chew; Six Cohorts: B.F. Zhou, H.Y. Zhang; Tanno/Soubetsu: K. Shimamoto, S. Saitoh; Tianjin: Z.Z. Li, H.Y. Zhang; Western Australia AAA Screenees: P. Norman, K. Jamrozik; Xi’an: Y. He, T.H. Lam; Yunnan: S.X. Yao.
References
- Ferlay, J; Bray, F; Pisani, P; Parkin, DM. GLOBOCAN 2002, Cancer Incidence, Mortality and Prevalence Worldwide; IARC Cancer Base No 5 Version 20,IARC Press: Lyon, France, 2004. [Google Scholar]
- Parkin, DM; Bray, F; Ferlay, J; Pisani, P. Global cancer statistics, 2002. C.A. Cancer J. Clin 2005, 55, 74–108. [Google Scholar]
- Gluckman, JL; Farrell, M. Internal Medicine, 5th Ed; Stein, JH, Ed.; Mosby Inc: St, Louis, USA, 1998; Chapter 98. [Google Scholar]
- Schottenfeld, D; Fraumeni, F. Cancer epidemiology and prevention, 3rd Ed ed; Oxford University Press: New York, USA, 2006. [Google Scholar]
- Ezzati, M; Henley, SJ; Lopez, AD; Thun, MJ. Role of smoking in global and regional cancer epidemiology, current patterns and data needs. Int. J. Cancer 2005, 116, 963–971. [Google Scholar]
- World Health Organization. WHO Global Status Report on Alcohol 2004; WHO: Geneva, Switzerland.
- Zeka, A; Gore, R; Kriebel, D. Effects of alcohol and tobacco on aero digestive cancer risks, a meta-regression analysis. Cancer Cause. Control 2003, 14, 897–906. [Google Scholar]
- Jha, P; Paccaud, F; Nguyen, S. Tobacco control in developing countries Curbing the epidemic; Chapter 19. World Bank, Oxford University Press: New York, USA, 2000. [Google Scholar]
- Yang, G; Fan, L; Tan, J; Qi, G; Zhang, Y; Samet, JM; Taylor, CE; Becker, K; Xu, J. Smoking in China, findings of the 1996 National Prevalence Survey. JAMA 1999, 282, 1247–1253. [Google Scholar]
- Martiniuk, AL; Lee, CM; Lam, TH; Huxley, R; Suh, I; Jamrozik, K; Gu, DF; Woodward, M. The fraction of ischaemic heart disease and stroke attributable to smoking in the WHO Western Pacific and South-East Asian regions. Tob. Control 2006, 15, 181–188. [Google Scholar]
- Samet, JM; Yoon, S-Y. Women and the Tobacco Epidemic; World Health Organisation: Geneva, Switzerland, 2001. [Google Scholar]
- Woodward, M; Barzi, F; Martiniuk, A; Fang, X; Gu, DF; Imai, Y; Lam, TH; Pan, WH; Rodgers, A; Suh, I; Jee, SH; Ueshima, H; Huxley, R. Cohort profile, The Asia Pacific Cohort Studies Collaboration. Int. J. Epidemiol 2006, 35, 1412–1416. [Google Scholar]
- Woodward, M. Epidemiology, study design and data analysis, 2nd Ed ed; Chapman and Hall/CRC Press: Boca Raton, USA, 2005. [Google Scholar]
- Bagnardi, V; Blangiardo, M; La Vecchia, C; Corrao, G. Alcohol consumption and the risk of cancer, a meta-analysis. Alcohol Res. Health 2001, 25, 263–270. [Google Scholar]
- Nordlund, LA; Carstensen, JM; Pershagen, G. Cancer incidence in female smokers, a 26-year follow-up. Int. J. Cancer 1997, 73, 625–628. [Google Scholar]
- Lindblad, M; Rodriguez, LA; Lagergren, J. Body mass, tobacco and alcohol and risk of esophageal, gastric cardia, and gastric non-cardia adenocarcinoma among men and women in a nested case-control study. Cancer Cause. Control 2005, 16, 285–294. [Google Scholar]
- Jiang, JM; Zeng, XJ; Chen, JS; Ping, Z; Li, JY; Zhang, KL; Wu, YP; Liu, BQ. Smoking and mortality from esophageal cancer in China: a large case-control study of 19,734 male esophageal cancer deaths and 104,846 living spouse controls. Int. J. Cancer 2006, 119, 1427–1432. [Google Scholar]
- Sakata, K; Hoshiyama, Y; Morioka, S; Hashimoto, T; Takeshita, T; Tamakoshi, A. Smoking, alcohol drinking and esophageal cancer, findings from the JACC Study. J. Epidemiol 2005, 15, 212–219. [Google Scholar]
- Jee, SH; Samet, JM; Ohrr, H; Kim, JH; Kim, IS. Smoking and cancer risk in Korean men and women. Cancer Cause. Control 2004, 15, 341–348. [Google Scholar]
- McLaughlin, JK; Hrubec, Z; Blot, WJ; Fraumeni, JF, Jr. Smoking and cancer mortality among U.S. veterans, a 26-year follow-up. Int. J. Cancer 1995, 60, 190–193. [Google Scholar]
- La Vecchia, C; Bidoli, E; Barra, S; D'Avanzo, B; Negri, E; Talamini, R; Franceschi, S. Type of cigarettes and cancers of the upper digestive and respiratory tract. Cancer Cause. Control 1990, 1, 69–74. [Google Scholar]
- Bosetti, C; Gallus, S; Franceschi, S; Levi, F; Bertuzzi, M; Negri, E; Talamini, R; La Vecchia, C. Cancer of the larynx in non-smoking alcohol drinkers and in non-drinking tobacco smokers. Br. J. Cancer 2002, 87, 516–518. [Google Scholar]
- Castellsague, X; Quintana, MJ; Martinez, MC; Nieto, A; Sánchez, MJ; Juan, A; Monner, A; Carrera, M; Agudo, A; Quer, M; Muñoz, N; Herrero, R; Franceschi, S; Bosch, FX. The role of type of tobacco and type of alcoholic beverage in oral carcinogenesis. Int. J. Cancer 2004, 108, 741–749. [Google Scholar]
- Lopez, AD; Collishaw, NE; Piha, T. A descriptive model of the cigarette epidemic in developed countries. Tob. Control 1994, 3, 242–247. [Google Scholar]
- Wynder, E; Fujita, Y; Harris, RE; Hirayama, T; Hiyama, T. Comparative epidemiology of cancer between the United States and Japan, a second look. Cancer 1991, 67, 746–763. [Google Scholar]
- Peto, R; Chen, ZM; Boreham, J. Tobacco – the growing epidemic. Nature Med 1999, 5, 15–17. [Google Scholar]
- Zhang, H; Cai, B. The impact of tobacco on lung health in China. Respirology 2003, 8, 17–21. [Google Scholar]
- Ando, M; Wakai, K; Seki, N; Tamakoshi, A; Suzuki, K; Ito, Y; Nishino, Y; Kondo, T; Watanabe, Y; Ozasa, K; Ohno, Y. Attributable and absolute risk of lung cancer death by smoking status. Findings from the Japan Collaborative Cohort Study. Int. J. Cancer 2003, 105, 249–254. [Google Scholar]
- Marugame, T; Sobue, T; Satoh, H; Komatsu, S; Nishino, Y; Nakatsuka, H; Nakayama, T; Suzuki, T; Takezaki, T; Tajima, K; Tominaga, S. Lung cancer death rates by smoking status. Comparison of the Three-Prefecture Cohort study in Japan to the Cancer Prevention Study II in the USA. Cancer Sci 2005, 96, 120–126. [Google Scholar]
- Pintos, J; Franco, EL; Kowalski, LP; Oliveira, BV; Curado, MP. Use of wood stoves and risk of cancers of the upper aero-digestive tract, a case-control study. Int. J. Epidemiol 1998, 27, 936–940. [Google Scholar]
- Ministry of Health, Peoples’ Republic of China. 2007 China Tobacco Control Report-Create a Smoke-Free Environment; Enjoy a Healthy Life; Office of the Leading Small Group for Implementation of the Framework Convention on Tobacco Control: Beijing, China, May 2007. [Google Scholar]
- Ide, R; Mizoue, T; Fujino, Y; Hoshiyama, Y; Sakata, K; Tamakoshi, A; Yoshimura, T. Cigarette smoking, alcohol drinking, and oral and pharyngeal cancer mortality in Japan. Oral Dis 2008, 14, 314–319. [Google Scholar]
- Nilsson, S; Carstensen, JM; Pershagen, G. Mortality among male and female smokers in Sweden, a 33 year follows up. J. Epidemiol. Community Health 2001, 55, 825–830. [Google Scholar]
- Zang, EA; Wynder, EL. Differences in lung cancer risk between men and women, examination of the evidence. J. Natl. Cancer Inst 1996, 88, 183–192. [Google Scholar]
- Osann, KE; Anton-Culver, H; Kurosaki, T; Taylor, T. Sex differences in lung-cancer risk associated with cigarette smoking. Int. J. Cancer 1993, 54, 44–48. [Google Scholar]
- Wynder, EL; Muscat, JE. The changing epidemiology of smoking and lung cancer histology. Environ. Health Perspect 1995, 8, 143–148. [Google Scholar]
- Gao, YT; Blot, WJ; Zheng, W; Ershow, AG; Hsu, CW; Levin, LI; Zhang, R; Fraumeni, JF, Jr. Lung cancer among Chinese women. Int. J. Cancer 1987, 40, 604–609. [Google Scholar]
- Taioli, E; Wynder, EL. Endocrine factors and adenocarcinoma of the lung in women. J. Natl. Cancer Inst 1994, 86, 869–870. [Google Scholar]
- Bain, C; Feskanich, D; Speizer, FE; Thun, M; Hertzmark, E; Rosner, BA; Colditz, GA. Lung cancer rates in men and women with comparable histories of smoking. J. Natl. Cancer Inst 2004, 96, 826–834. [Google Scholar]
- Mackay, J; Eriksen, M; Shafey, O. The Tobacco Atlas, 2nd Ed ed; American Cancer Society: Atlanta, Georgia, USA, 2006. [Google Scholar]
- Bosetti, C; Gallus, S; Garavello, W; La Vecchia, C. Smoking cessation and the risk of oesophageal cancer. An overview of published studies. Oral Oncol 2006, 42, 957–964. [Google Scholar]



| Country
| Study
| Baseline
| No. of subjects
| Mean age (yrs)
| Female (%)
| Current drinker s %
| Current smokers (%)
| Former smoker s (%)
| Mean cigarettes per day
| Media n FU (yrs)
| UADT cancer deaths
| ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M
| F
| M
| F
| M
| F
| M
| F
| ||||||||
| Australia | Busselton | 1966–81 | 7789 | 44.9 | 52 | 81 | 57 | 44 | 24 | 25 | 12 | 19 | 16 | 26.5 | 34 |
| Australia | Long. Study of Aging | 1992–93 | 1610 | 78.1 | 48 | 71 | 58 | 8 | 8 | 62 | 23 | 16 | 13 | 4.6 | 3 |
| Australia | National Heart Foundation | 1989–90 | 9277 | 43.5 | 51 | 87 | 75 | 27 | 21 | 32 | 20 | 21 | 16 | 8.3 | 12 |
| Australia | Newcastle | 1983–94 | 5929 | 51.7 | 50 | 85 | 65 | 28 | 18 | 37 | 18 | 20 | 18 | 8.9 | 13 |
| Australia | Perth | 1978–94 | 10230 | 45.0 | 48 | 90 | 75 | 30 | 21 | 31 | 18 | 20 | 16 | 14.4 | 9 |
| Australia | WA AAA Screenees | 1996–99 | 12203 | 72.2 | 0 | 82 | - | 11 | - | 60 | - | 14 | - | 3.2 | 22 |
| Fletcher | 1992–94 | 10326 | 44.3 | 28 | 87 | 77 | 26 | 18 | 33 | 28 | 15 | 13 | 5.8 | 4 | |
| NZ
| Challenge
| ||||||||||||||
| ANZ | Subtotal | 1966–99 | 57364 | 52.0 | 35 | 84 | 69 | 23 | 20 | 42 | 19 | 18 | 15 | 7.9 | 97 |
| China | Anzhen | 1991 | 8378 | 53.8 | 55 | 39 | 3 | 51 | 10 | 9 | 2 | 15 | 10 | 4.3 | 5 |
| China | Fangshan Guangzhou | 1991–92 | 2619 | 47.3 | 67 | 43 | 2 | 75 | 22 | 6 | 2 | 15 | 9 | 3.6 | 1 |
| China | Occupational Seven Cities | 1985–98 | 166695 | 41.5 | 22 | 27 | 4 | 60 | 1 | 1 | 0 | 15 | 12 | 7.3 | 146 |
| China | Cohorts | 1987 | 10811 | 53.9 | 55 | 42 | 6 | 57 | 17 | 8 | 2 | - | - | 2.7 | 11 |
| China | Yunnan | 1992 | 6581 | 55.8 | 3 | 85 | 70 | 70 | 0 | 14 | 0 | 12 | - | 4.5 | 9 |
| Hong Kong | Hong Kong | 1985–91 | 2983 | 78.6 | 57 | 24 | 8 | 29 | 11 | 41 | 18 | 13 | 8 | 2.5 | 5 |
| Japan | Akabane | 1985–86 | 1834 | 54.5 | 56 | 62 | 5 | 62 | 1 | 21 | 0 | 23 | 9 | 11.0 | 1 |
| Japan | Civil Service Workers | 1990–92 | 9240 | 46.7 | 33 | 82 | 48 | 51 | 11 | 26 | 3 | - | - | 6.7 | 3 |
| Japan | Hisayama | 1961 | 1601 | 56.1 | 56 | 70 | 8 | 76 | 17 | 4 | 1 | - | - | 24.6 | 4 |
| Japan | Konan | 1987–95 | 1226 | 51.7 | 55 | 78 | 23 | 62 | 5 | 14 | 1 | 22 | 11 | 6.4 | 3 |
| Japan | Ohasama | 1992–93 | 2240 | 59.5 | 64 | 65 | 8 | 51 | 2 | 11 | 0 | - | - | 4.1 | 2 |
| Japan | Saitama | 1986–90 | 3615 | 54.5 | 62 | 77 | 33 | 63 | 8 | 21 | 2 | 22 | 13 | 11.0 | 9 |
| Japan | Shibata | 1977 | 2350 | 56.9 | 58 | 72 | 9 | 72 | 4 | 6 | 0 | 20 | 10 | 20.0 | 10 |
| Japan | Shigaraki Town | 1991–97 | 3730 | 57.1 | 59 | 70 | 19 | 59 | 8 | 22 | 2 | 22 | 13 | 4.4 | 4 |
| Singapore | Singapore Heart | 1982–97 | 2321 | 40.7 | 49 | 50 | 18 | 41 | 3 | 14 | 1 | - | - | 14.6 | 1 |
| Singapore | Singapore NHS92 | 1992 | 3305 | 39.2 | 52 | 48 | 15 | 35 | 3 | 12 | 0 | - | - | 6.2 | 3 |
| S. Korea | KMIC | 1992 | 160242 | 44.0 | 33 | 73 | 9 | 58 | 0 | 21 | 0 | - | - | 4.0 | 49 |
| Taiwan | CVDFACTS | 1988–96 | 5729 | 47.2 | 55 | 16 | 1 | 48 | 1 | 7 | 0 | - | - | 6.0 | 2 |
| Taiwan
| Kinmen
| 1993–97
| 2545
| 63.2
| 49
| 44
| 4
| 50
| 5
| 17
| 1
| -
| -
| 2.9
| 6
|
| Asia | Subtotal | 1961–98 | 398045 | 44.6 | 32 | 49 | 9 | 59 | 5 | 11 | 1 | 15 | 11 | 5.3 | 274 |
| Total | 1961–99 | 455409 | 46.0 | 32 | 53 | 17 | 55 | 7 | 14 | 3 | 16 | 14 | 5.9 | 371 | |
| Cancer type | No. of subjects | No. of deaths | Adjusted for age HR and 95 % CI | Adjusted for age & alcohol HR and 95 % CI |
|---|---|---|---|---|
| Lip, oral cavity or pharynx | ||||
| Never-smoked | 142700 | 57 | 1 | 1 |
| <20 CPD | 50077 | 34 | 1.83 (1.14–2.95) | 1.86 (1.15–3.00) |
| ≥20 CPD
| 38785
| 43
| 2.32 (1.47–3.65)
| 2.37 (1.49–3.78)
|
| P for trend
| <0.001
| <0.001
| ||
| Esophagus | ||||
| Never-smoked | 142700 | 33 | 1 | 1 |
| <20 CPD | 50077 | 17 | 2.74 (1.37–5.49) | 2.52 (1.25–5.07) |
| ≥20 CPD
| 38785
| 25
| 3.89 (1.99–7.60)
| 3.40 (1.71–6.76)
|
| P for trend
| <0.001
| <0.001
| ||
| Larynx | ||||
| Never-smoked | 142700 | 7 | 1 | 1 |
| <20 CPD | 50077 | 2 | 1.18 (0.22–6.17) | 1.28 (0.24–6.76) |
| ≥20 CPD | 38785 | 9 | 5.25 (1.62–17.0) | 5.91 (1.81–19.3) |
| P for trend
| 0.005
| 0.003
| ||
| Overall | ||||
| Never-smoked | 142700 | 97 | 1 | 1 |
| <20 CPD | 50077 | 53 | 2.03 (1.38–2.97) | 2.01 (1.37–2.95) |
| ≥20 CPD
| 38785
| 77
| 2.88 (2.01–4.11)
| 2.83 (1.96–4.09)
|
| P for trend | <0.001 | <0.001 | ||
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ansary-Moghaddam, A.; Martiniuk, A.; Lam, T.-H.; Jamrozik, K.; Tamakoshi, A.; Fang, X.; Suh, I.; Barzi, F.; Huxley, R.; Woodward, M. Smoking and the Risk of Upper Aero Digestive Tract Cancers for Men and Women in the Asia-Pacific Region. Int. J. Environ. Res. Public Health 2009, 6, 1358-1370. https://doi.org/10.3390/ijerph6041358
Ansary-Moghaddam A, Martiniuk A, Lam T-H, Jamrozik K, Tamakoshi A, Fang X, Suh I, Barzi F, Huxley R, Woodward M. Smoking and the Risk of Upper Aero Digestive Tract Cancers for Men and Women in the Asia-Pacific Region. International Journal of Environmental Research and Public Health. 2009; 6(4):1358-1370. https://doi.org/10.3390/ijerph6041358
Chicago/Turabian StyleAnsary-Moghaddam, Alireza, Alexandra Martiniuk, Tai-Hing Lam, Konrad Jamrozik, Akiko Tamakoshi, Xianghua Fang, Il Suh, Federica Barzi, Rachel Huxley, and Mark Woodward. 2009. "Smoking and the Risk of Upper Aero Digestive Tract Cancers for Men and Women in the Asia-Pacific Region" International Journal of Environmental Research and Public Health 6, no. 4: 1358-1370. https://doi.org/10.3390/ijerph6041358
APA StyleAnsary-Moghaddam, A., Martiniuk, A., Lam, T.-H., Jamrozik, K., Tamakoshi, A., Fang, X., Suh, I., Barzi, F., Huxley, R., & Woodward, M. (2009). Smoking and the Risk of Upper Aero Digestive Tract Cancers for Men and Women in the Asia-Pacific Region. International Journal of Environmental Research and Public Health, 6(4), 1358-1370. https://doi.org/10.3390/ijerph6041358