Advances in Identifying Beryllium Sensitization and Disease
Abstract
:1. Background
1.1. Early Disease Reports
1.2. The Beryllium Registry
- lower respiratory symptoms;
- reticulonodular infiltrates on chest x-ray;
- restrictive or obstructive pulmonary impairment, or depressed diffusing capacity;
- biopsy showing non-caseating granulomas or mononuclear cell interstitial infiltrates.
1.3. Exposure and Susceptibility
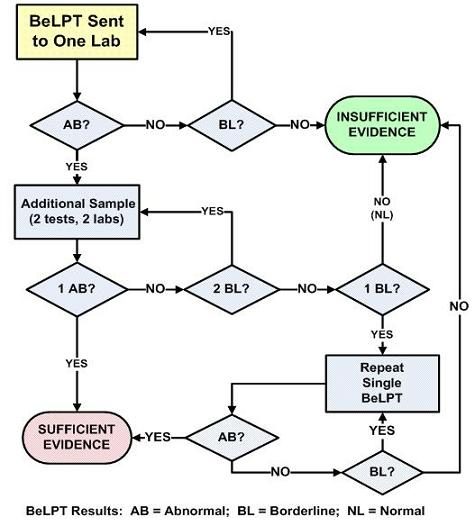
1.4. The BeLPT
- normal test result (NL)—0 of 6 incubations with Be are elevated;
- borderline test result (BL)—1 of 6 incubations with Be are elevated; and,
- abnormal test result (AB)—2 or more incubations with Be are elevated.
1.5. Test-Retest Inconsistencies
2. Introduction
2.1. The Expert Panel
- one abnormal result,
- one abnormal and one borderline result, and,
- two abnormal results.
2.2. Confusing Issues
- Sensitivity—the proportion of persons with BeS that test abnormal;
- Specificity—the proportion of persons without BeS who test normal;
- Positive predictive value (PPV) – the proportion of persons testing abnormal who are truly BeS.
3. Probability Modeling
- if a true positive occurs, additional testing will confirm it; and,
- if a false positive occurs, additional testing will not confirm it.
3.1. Building a Foundation
3.2. Probability Modeling
3.3. The National Research Council (NRC)
“The BeLPT is integral to any screening program. No alternative tests have been adequately validated to be put into practice outside research settings.”
4. Validation
4.1. DOE Pre-hires at Rocky Flats
4.2. Manatee County, FL
4.3. Japan
4.4. Ottawa County, OH
5. Conclusions
5.1. Modeling vs. Measurement
5.2. Not Truly Sensitized
5.3. Truly Sensitized
References
- Cunningham, LD. Beryllium Recycling in the United States in 2000.
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Beryllium.
- Gelman, I. Poisoning by vapors of beryllium oxyfluoride. J. Indust. Hyg. Toxicol. 1936, 18, 371–379. [Google Scholar]
- Cummings, KJ; Stefaniak, AB; Virji, MA; Kreiss, K. A reconsideration of acute beryllium disease. Environ. Health Perspect. 2009, 117, 1250–1256. [Google Scholar]
- Sprince, NL. Beryllium disease. In Occupational Respiratory Diseases; Merchant, JA, Ed.; National Institute for Occupational Safety and Health: Washington, DC, USA, 1986; pp. 385–399. [Google Scholar]
- Middleton, DC. Chronic beryllium disease: Uncommon disease, less common diagnosis. Environ. Health Perspect. 1998, 106, 765–767. [Google Scholar]
- Newman, LS; Kreiss, K; King, TE; Seay, S; Campbell, PA. Pathologic and immunologic alterations in early stages of beryllium disease. Re-examination of disease definition and natural history. Am. Rev. Respir. Dis. 1989, 139, 1479–1486. [Google Scholar]
- McCawley, MA; Kent, MS; Berakis, MT. Ultrafine beryllium number concentration as a Possible metric for chronic beryllium disease risk. Appl. Occup. Environ. Hyg. 2001, 16, 631–638. [Google Scholar]
- Stefaniak, AB; Hoover, MD; Day, GA; Dickerson, RM; Peterson, EJ; Kent, MS; Schuler, CR; Breysse, PN; Scripsick, RC. Characterization of physicochemical properties of beryllium aerosols associated with prevalence of chronic beryllium disease. Environ. Monit. 2004, 6, 523–532. [Google Scholar]
- Tinkle, SS; Antonini, JM; Rich, BA; Roberts, JR; Salmen, R; DePree, K; Adkins, EJ. Skin as a route of exposure and sensitization for chronic beryllium disease. Environ. Health Perspect. 2003, 111, 1202–1208. [Google Scholar]
- Henneberger, PK; Goe, SK; Miller, WE; Doney, B; Groce, DW. Industries in the United States with airborne beryllium exposure and estimates of the number of current workers potentially exposed. J. of Occup. and Environ. Hyg. 2004, 1, 648–659. [Google Scholar]
- Kreiss, K; Day, GA; Schuler, CR. Beryllium: A Modern Industrial Hazard. Annu. Rev. Public Health 2007, 28, 259–277. [Google Scholar]
- Richeldi, L; Sorrentino, R; Saltini, C. HLA-DPB1 glutamate 69: a genetic marker of beryllium disease. Science 262, 242–244.
- Deubner, DC; Goodman, M; Iannuzzi, J. Variability, predictive value, and uses of the beryllium blood lymphocyte proliferation test (BLPT): Preliminary analysis of the ongoing workforce survey. Appl. Occup. Environ. Hyg. 2001, 16, 521–526. [Google Scholar]
- Middleton, DC; Lewin, MD; Kowalski, PJ; Cox, SS; Kleinbaum, D. The BeLPT: Algorithms and interpretations. Am. J. Ind. Med. 2006, 49, 36–44. [Google Scholar]
- Stange, AW; Furman, FJ; Hilmas, DE. The beryllium lymphocyte proliferation test: Relevant issues in beryllium health surveillance. Am. J. Ind. Med. 2004, 46, 453–462. [Google Scholar]
- Middleton, DC; Fink, J; Kowalski, PJ; Lewin, MD; Sinks, T. Optimizing the BeLPT criteria for beryllium sensitization. Am. J. Ind. Med. 2008, 51, 166–172. [Google Scholar]
- NRC (National Research Council) of the National Academies. Managing health effects of beryllium exposure. The National Academies Press: Washington, DC, USA, 2008; p. 168. [Google Scholar]
- Donovan, EP; Kolanz, ME; Galbraith, DA; Chapman, PS; Paustenbach, DJ. Performance of the beryllium blood lymphocyte proliferation test based on a long-term occupational surveillance program. Int. Arch. Occup. Environ. Health. 2007, 81, 165–178. [Google Scholar]



| Results that Meet Criteria2 | Test 1 | Test 2 | Test 3 | Outcome Probability p = p1 * p2 * p3 |
|---|---|---|---|---|
| Combination 1 | abnormal | --- | --- | 0.5970 |
| Combination 2 | borderline | abnormal | --- | 0.0752 |
| Combination 3 | borderline | borderline | abnormal | 0.0095 |
| Overall likelihood of meeting the criteria of one abnormal | 0.6817 | |||
| Sensitization Criteria | Sensitivity | Specificity | PPV at4% BeS 2 |
|---|---|---|---|
| 1 AB | 0.682 | 0.9889 | 0.719 |
| 1 AB + 1 BL | 0.657 | 0.9992 | 0.972 |
| 2 AB | 0.612 | 0.9998 | 0.992 |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Middleton, D.; Kowalski, P. Advances in Identifying Beryllium Sensitization and Disease. Int. J. Environ. Res. Public Health 2010, 7, 115-124. https://doi.org/10.3390/ijerph7010115
Middleton D, Kowalski P. Advances in Identifying Beryllium Sensitization and Disease. International Journal of Environmental Research and Public Health. 2010; 7(1):115-124. https://doi.org/10.3390/ijerph7010115
Chicago/Turabian StyleMiddleton, Dan, and Peter Kowalski. 2010. "Advances in Identifying Beryllium Sensitization and Disease" International Journal of Environmental Research and Public Health 7, no. 1: 115-124. https://doi.org/10.3390/ijerph7010115
APA StyleMiddleton, D., & Kowalski, P. (2010). Advances in Identifying Beryllium Sensitization and Disease. International Journal of Environmental Research and Public Health, 7(1), 115-124. https://doi.org/10.3390/ijerph7010115