2. Human Exposure to Polycyclic Aromatic Hydrocarbons (PAHs)
Human exposure to combustion products and mixtures containing carcinogenic PAHs has long been associated with cancer induction [
14]. Over 200 years ago it was reported that chimney sweeps, who often worked unclothed and bathed rarely, were highly susceptible to cancer of the scrotum. Subsequently, bathing as a deterrent to cancer induction became an early successful intervention, confirming the association between soot exposure and scrotal cancer risk [
3]. By the late 1970s PAH-DNA adduct formation was well documented in experimental models, primarily through the use of radiolabeled compounds. Non-invasive methods to measure DNA damage in human tissues had yet to be developed [
15]. Investigators recognized that in order to extend knowledge of mechanistic cancer etiology in humans it would be necessary to develop highly-sensitive, non-invasive methods of DNA adduct measurement. The development of antisera specific for carcinogen-DNA adducts or carcinogen-modified DNA was one such approach that allowed for DNA adduct determination using immunohistochemistry of archived tissue as well as immunoassay of extracted DNA [
16].
3. Use of Immunoassay and Immunohistochemistry for Human PAH-DNA Adduct Detection
To examine PAH-DNA adducts in human tissues, investigators elicited a rabbit antiserum against DNA modified with an activated form of benzo[
a]pyrene (BP), the r7,t8-dihydroxy-t-9, 10-oxy-7,8,9,10-tetrahydrobenzo[
a]pyrene (BPDE) [
17]. The immunogen DNA contained a single adduct, the r7,t8,t9-trihydroxy-c-10-(N
2deoxyguanosyl)-7,8,9,10-tetrahydrobenzo[
a]pyrene (BPdG) adduct. However, the resulting antiserum cross-reacted with DNA samples modified with diol-epoxides of additional PAHs including: chrysene, benzo[
k]fluoranthene, dibenz[
a,c]anthracene, and the bay-region and non-bay region benz[
a]anthracenes [
18]. Individually, these compounds are carcinogenic in animal models and have been classified by the US Environmental Protection Agency (US EPA) and the International Agency for Research on Cancer (IARC) as either known or probable human carcinogens [
19]. Because most human PAH exposures are to complex mixtures, human DNA samples are presumed to contain multiple PAH-DNA adducts, many of which will be detected by the BPDE-DNA antiserum. The resulting increased sensitivity may be advantageous for biomonitoring and measuring DNA damage induced by a family of carcinogenic PAHs. Where nomenclature is concerned, the BPDE-DNA immunoassays measure only BPdG in samples from animals exposed experimentally to BP, but when used with human samples the results are termed “PAH-DNA adducts” because human DNA likely contains adducts of multiple carcinogenic PAHs.
3.1. Validation of PAH-DNA Measurements by Immunoassay
Over the years many studies have contributed to validation of PAH-DNA adduct measurements in human DNA using immunoassays, the most-sensitive of which is the chemiluminescence immunoassay (CIA) [
20]. These studies have shown that human blood cell PAH-DNA adducts result from dietary ingestion of PAHs [
21], increase or decrease as a function of the quantity of PAHs ingested [
22], and have a half-life of about a week in human blood [
23–
25]. In wildland firefighters, variations in blood cell PAH-DNA adducts correlated with recent charbroiled meat consumption and not recent firefighting activity [
24,
25]. When PAH levels in the diet are low, inhalation can be a significant source of PAH exposure. A cohort study of US Army soldiers who were moved from an area with a clean ambient environment to one that was much more polluted revealed significant increases in blood cell PAH-DNA adduct levels for each individual [
26,
27]. Similarly, a significantly higher level of blood cell PAH-DNA adducts was found in young adults sampled in Mexico City in the dry season, when airborne PAH levels were higher, compared to the rainy season, when PAH levels were lower [
28]. Using samples from the Navy Colon Adenoma study we found a 3-fold increased risk of colorectal adenoma in the quartile of individuals with the highest leukocyte PAH-DNA adducts, compared to the quartile with the lowest PAH-DNA adducts [
29]. These were, coincidentally, the same individuals who consistently ingested the largest quantities of heavily-cooked beef [
30], suggesting a causal association between ingestion of well-cooked beef, PAH-DNA adduct formation and adenoma risk. Taken together, all of the above studies suggest that PAH-DNA adduct levels vary with PAH exposures, and that reducing PAH-DNA levels might also decrease human cancer risk.
3.2. Localization and Semi-Quantitation of PAH-DNA Adducts by Immunohistochemistry (IHC)
We have had a long-term interest in the use of IHC for localization of PAH-DNA adducts using the same antiserum employed for immunoassays (above). Using IHC, tissue morphology is retained and there is the potential to reveal “target” cells, those having the highest concentration of DNA adducts. In addition, molecular epidemiology studies require large numbers of samples, and archived paraffin blocks are a ready source of such materials. In this review we will describe studies using the BPDE-DNA antiserum to localize and semi-quantify PAH-DNA adducts in multiple human tissues, including esophagus, prostate, cervix, vulva, and placenta.
All of the IHC studies to be described here involved automated staining (using Ventana NexES) of paraffin-embedded tissues with the BPDE-DNA antiserum, and the use of a Fast Red—conjugated secondary antiserum to reveal adducted (pink) nuclei [
31]. Hematoxylin staining of an adjacent tissue section revealed the total number of nuclei in the area chosen for evaluation, and the staining of an additional adjacent tissue section with BPDE-DNA antiserum absorbed with the immunogen, BPDE-DNA, provided a negative control, which demonstrated the specificity of the PAH-DNA signal. The Automated Cellular Imaging System (ACIS; Chromavision, Inc.), which has the capacity to automate, digitize and expedite pathology evaluations [
32,
33], was used to score DNA adducts in tissue nuclei. Following capture of a high-resolution digital image, operator-defined regions were evaluated according to study-specific parameters of color/hue (red-pink) and object/nucleus size and shape. The resulting ACIS-discerned nuclear color intensity was reported as Optical Density (OD). Identical regions in adjacent hemotoxylin-stained sections were evaluated to provide a count of nuclei for each respective region. The resulting OD/nucleus values were then converted to units of adducts/10
8 nucleotides by comparing the unknown samples to a standard curve.
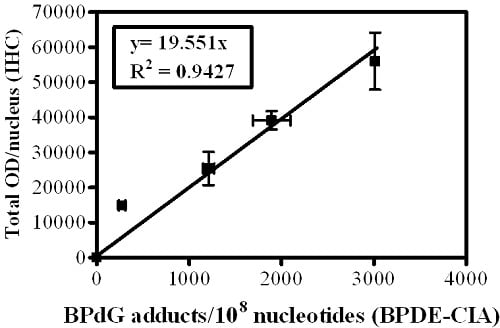
Cultured human keratinocytes exposed to incremental concentrations of BPDE were separated into two portions. Half of the BPDE-exposed human cells were fixed in formalin, embedded in paraffin then stained for PAH-DNA adducts by IHC (
Figure 1A). Cells stained with specific and immunogen-absorbed antiserum are shown, respectively, in the top and bottom rows of
Figure 1A. Inserts contain staining intensity values (OD/nucleus) for the pink color determined by ACIS. DNA was extracted from the remaining BPDE-exposed human cells and quantitatively analyzed for BP-DNA adducts by BPDE-DNA CIA (
Figure 1B). The standard curve (
Figure 1C) comprises a graph in which OD/nucleus values (
Figure 1A) are plotted as a function of the BPdG adducts/10
8 nucleotides values determined by CIA (
Figure 1B). Values represent mean ± range for two CIAs (each with triplicate wells), and mean ± range for two IHC arrays, with staining of three cores and three regions/core for each experimental group (Adapted from John,
et al., 2009 [
34]). The resulting values for PAH-DNA adducts/10
8 nucleotides in unknown samples are
semi-quantitative because of the uncertain composition of PAH adducts in human DNA. In addition, the numbers generated for PAH-DNA adducts/10
8 nucleotides by IHC/ACIS are substantially higher than those measured when DNA is extracted from whole tissue and adducts are measured by BPDE-DNA CIA. This occurs largely because the IHC methodology allows us to choose a specific, highly-modified target area for evaluation, whereas, for CIA measurements, the extracted DNA is from all cells in the sample tissue.
4. Human Esophagus
Linxian (Linxou), China, is located in a mountainous region where indoor coal stoves lacking external venting are used for cooking and heating and the cumulative mortality rate from esophageal and proximal stomach cancer is among the highest in the world. In this area, high levels of carcinogenic PAHs, including BP, have been detected in raw and cooked food [
35]. The tissues examined in our study were biopsies collected from patients for the purpose of diagnosing squamous carcinoma-
in situ or early invasive squamous cell carcinoma. The samples were obtained and paraffin-embedded in 1985 [
31] and 1994 [
36]. Because of the age of the samples, we discarded the first several cuts off each paraffin block in order to perform the staining on non-oxidized tissue.
Figure 2 shows samples of human esophagus taken from Linxian in 1985 and 1994 and stained up to 17 years later with: hematoxylin (
Figure 2A and D); immunogen-absorbed BPDE-DNA antiserum (
Figure 2B and E, shows specificity of antiserum); and specific BPDE-DNA antiserum (
Figure 2C and F). In
Figure 2C it is evident that PAH-DNA adducts concentrate in the nuclei of the esophageal epithelium, especially in the vicinity of the basal cell layer. For comparison, and to validate the methodology, esophageal tissue was collected at autopsy from 6 individuals in the US who died from conditions unrelated to esophageal cancer. Staining of these tissues (not shown) revealed no evidence of PAH-DNA adduct formation [
31]. Taken together, these studies indicate that tissues archived for many years can successfully be queried for the presence of PAH-DNA adducts as exposure biomarkers. In addition, it appears that the population of Linxian is exposed to PAHs through the ambient air and the food supply, and both sources of exposure likely contribute to the observed PAH-DNA adducts. The presence of PAH-DNA adducts in esophagus is consistent with the epidemiological finding that esophageal tumor incidence is high, however, a causal association between PAH exposure, PAH-DNA adduct formation and esophageal cancer risk remains to be established.
5. Human Prostate
The etiology of prostatic adenocarcinoma is interesting in that the tumors arise almost exclusively in the peripheral zone (PZ) of the prostate gland, whereas the transition zone (TZ) tissue develops occasional benign hypertrophy, but no tumors. In Western countries an increased risk of adenocarcinoma of the prostate is associated with diet and lifestyle factors, including smoking. Therefore, we hypothesized that if PAH-DNA adduct formation played a role in prostate cancer etiology, the PZ might have higher levels of adducts than the TZ. To investigate this we examined PAH-DNA adduct formation in non-tumorous portions of prostate tissue collected from 23 men undergoing either radical retropubic prostatectomy for low-volume prostatic adenocarcinoma (n = 22) or cystoprostectomy for bladder cancer (n = 1) [
34]. The patients were largely working-class, middle-aged men living in an industrialized area of the UK, with the majority reported to be smokers. Metabolic capacity, determined by expression studies of xenobiotic metabolizing enzymes, showed essentially similar metabolic capacity in PZ compared to TZ [
34], and normal appearing PZ and TZ areas were sectioned and examined by IHC/ACIS for PAH-DNA adducts (
Figure 3A–C).
Regardless of which zone was evaluated, the PAH-DNA adducts were localized primarily in the glandular epithelium (
Figure 3C). Overall, there was no significant difference in levels of PAH-DNA adducts localized in the PZ and the TZ, despite stratification by smoking status and diet. Using the standard curve shown in
Figure 1, PAH-DNA adduct values ranged from 8 adducts/10
8 nucleotides (the limit of detection, LOD), up to 1,812 and 2,214 adducts/10
8 nucleotides in the PZ and TZ, respectively [
34]. These were the highest values, and the most intensely-stained nuclei observed in any human tissue using the IHC/ACIS methodology (
Table 1). In fact, these values were approximately 10-fold higher than the PAH-DNA adducts observed using the same staining method for human cervix, vulva and placenta (
Table 1). We conclude that all zones of the human prostate sustain essentially similar PAH-DNA damage, which is primarily localized in the ductal epithelium. The particular susceptibility of the PZ to form tumors may result from the influence of PAH-DNA damage as well as other types of DNA damage and additional factors such as increased cell proliferation, inflammatory stimulation and epigenetic alterations. This study shows that prostate tissue is prone to high levels of PAH-DNA damage, but suggests that additional factors contribute to prostate cancer risk.
6. Human Cervix
In women infected with carcinogenic strains of the human papilloma virus (HPV), current and past smoking has been associated with a significantly increased risk of developing cervical cancer. We hypothesized that individuals with the higher concentrations of PAH-DNA adducts localized in the cervix may have the highest cancer risk, and that adduct formation would be associated with the womens’ smoking status. PAH-DNA adducts were evaluated in cervix tissue collected from HPV-infected women as part of a 10-year prospective study of factors that may increase the risk of developing cervical cancer [
37,
38]. We stained 78 coded samples, later revealed to consist of 29 cancer cases and 49 controls, and of these, 75 samples had sufficient normal-appearing epithelial tissue for IHC/ACIS analysis (
Figure 3D–F) [
38]. Localization of PAH-DNA adducts occurred throughout the cervix epithelium (
Figure 3F), though intensity of staining appeared to fade in the uppermost regions of the mature squamous epithelium. The largest nuclei and the highest PAH-DNA adduct concentration appeared in the basal layer, the region which was chosen for analysis (
Figure 3F). An average of 415 cells was counted for each sample and, using the standard curve (described above), the calculated PAH-DNA adduct values ranged from 20 (the LOD) to 191 adducts/10
8 nucleotides (
Table 1).
The data revealed a lack of correlation between DNA adduct formation and risk of cancer of the cervix. In addition, no correlation was found between PAH-DNA adduct formation and smoking status, stratified as never, former, low current (<10 cigarettes/day) and high current (>10 cigarettes/day) smoking. Unfortunately, the stratified sample groupings were small, and the demographic data did not reveal details of diet, second-hand smoke, or occupational PAH exposure. This study showed that PAH-DNA adducts are formed in human cervix with substantial (10-fold) interindividual variability, and are primarily localized in the basal cell layer of the cervix epithelium. The lack of a smoking correlation supports the notion that PAH-DNA damage formation, as determined by this staining approach, is not primarily a result of smoking. Literature data indicate that the alternate sources of PAH-exposure likely include ambient pollution, other inhaled products of organic combustion, and diet. The data presented here also suggest that carcinogenic components of cigarette smoke, other than the PAHs, may be responsible for the smoking-associated cancer risk in women infected with carcinogenic strains of HPV. Additional studies will be required to elucidate the relationship between smoking and cancer of the cervix.
7. Human Vulva
In a small pilot study, a series of human vulva biopsy samples, taken from non-involved areas, was obtained from 10 women being treated for vulvar intraepithelial neoplasia (VIN). We expected to find levels of PAH-DNA adducts in vulva similar to those observed in the cervix. Samples were evaluated by BPDE-DNA IHC/ACIS and comparison was made with the standard curve. The highest PAH-DNA adduct concentrations were found in the basal cells of the vulvar epithelium (
Figure 3G–I), similar to the localization observed in human cervix. All of the women examined had measurable PAH-DNA adducts, with values ranging from 8 (the LOD) to 206 adducts/10
8 nucleotides (
Figure 4). The vulva is susceptible to cancerous changes, which are less prevalent but otherwise not unlike those found in cervix. This pilot study demonstrated that PAH-DNA adducts are formed in vulvar epithelium (
Table 1), but a much larger study will be required to examine the contribution of PAH-DNA damage to risk of VIN.
8. Human Placenta
The placenta is not ordinarily considered a tissue subject to cancer risk, but it is a tissue of interest as an indicator of maternal and fetal exposure to carcinogens during pregnancy. We examined placenta samples obtained at birth following normal, full-term pregnancies in Teplice, an area of the Czech Republic historically known to have high levels of airborne pollution. In a long-term series of studies [
39,
40], children born in Teplice were found to have multiple health problems including intrauterine growth retardation, low birth weight and respiratory ailments. The object of our study was to determine PAH-DNA distribution in different cell types of the placenta and, if successful, to attempt quantification of the damage. We hypothesized that the metabolically-active cells would have the highest concentrations of PAH-DNA adducts. Placenta samples from seven smokers and seven non-smokers were stained and evaluated by IHC/ACIS [
41]. PAH-DNA adducts were concentrated in the cytotrophoblast cells and synctiotrophoblast knots lining the chorionic villi, finger-like projections which reach into the maternal blood (
Figure 5A). These cells, which are of fetal origin, are capable of metabolizing xenobiotics and it was, therefore, not a surprise that they contained highest concentrations of PAH-DNA adducts.
Figures 5B–D shows formalin-fixed paraffin-embedded human placenta samples collected and preserved immediately following normal, full-term pregnancies of women living in the Teplice region of the Czech Republic. Adjacent, parallel sections from a single placenta were stained with (B) hematoxylin, to obtain a count of total nuclei, (C) immunogen (BPDE-DNA)-absorbed serum, to demonstrate specificity of the serum, and (C) BPDE-DNA specific serum showing the presence of PAH-DNA adducts. The small circled areas indicate the ACIS-identified objects/nuclei within user-specified regions of interest.
By comparison with the BPDE-exposed human cervical keratinocyte standard curve, the level of PAH-DNA adducts in the placenta samples ranged from 49 to 312 PAH-DNA adducts/10
8 nucleotides, well above the LOD of 20 adducts/10
8 nucleotides [
41]. There was no significant difference in PAH-DNA adduct levels between smokers and non-smokers. However, the highest PAH-DNA adduct concentration found in placentas from Teplice, 312 adducts/10
8 nucleotides, was 1.5-fold higher than the highest levels found in human cervix and vulva (
Table 1) and consistent with the reports of high levels of ambient pollution in Teplice. We conclude that the metabolically competent cells of the Teplice placentas sustained high levels of PAH-DNA damage, reflective of significant PAH-exposures. The lack of char-broiled meat or fish in the local diet suggests that the primary source of this exposure may be from ambient air.
9. Methodological Considerations
The validity of this method as an indicator for cancer risk deserves mention. The antiserum is not specific for DNA damage induced by an individual PAH, but recognizes DNA adducts induced by a family of PAHs, many of which are either animal or human carcinogens, or both. Because most hydrocarbon exposures occur as complex mixtures, humans are exposed to everything contained in those mixtures. The BPDE-DNA antiserum detects DNA damage induced by several PAH carcinogens. Though less likely to form DNA adducts, this assay likely recognizes DNA damage induced by some non-carcinogens as well. Given that complex PAH mixtures typically contain PAH carcinogens, our assumption is that a positive PAH-DNA signal measured by the BPDE-DNA antiserum indicates a potential cancer-associated exposure. The PAH-DNA values generated by IHC/ACIS are semi-quantitative, and the numbers generated are 10–100 fold higher than those observed with BPDE-DNA CIA using extracted DNA. It is important to note, however, that IHC/ACIS values reflect investigator-selected areas, which are typically regions having the highest PAH-DNA adduct color intensity, whereas extraction of DNA from all the cells in a tissue results in effective dilution, as PAH-DNA adducts are not uniformly distributed to all tissues.
Several important technical challenges arose as these studies progressed. For example, in a complex tissue where adduct intensity may vary between cell types, it is important to decide which features of tissue architecture (
i.e., basal epithelium) will be examined so that consistency can be maintained throughout the analysis of a single group of samples. Also, it is important to evaluate cells in multiple (at least three or four) areas of a tissue section to incorporate as much of the intra-tissue variability as possible. In performing the placenta study [
41] we learned by experience that fresh tissue should be fixed and embedded immediately, and never frozen before paraffin blocks are made. The Linxian study [
31,
36] taught us that sectioned tissue on slides will oxidize over time, and it is therefore important to use fresh-cut tissue from an interior area of the paraffin block, not the first sections cut after storage of the block. Also, it is best to section the tissues no more than a couple of months before staining. Embedding the whole standard curve into a single block as a tissue array, so it can be placed on a single slide and stained simultaneously, has been a highly successful strategy that we owe to our collaborator, Stephen Hewitt [
42]. In addition, for the most accurate comparison, the standard curve array should be sectioned and stained at the same time as the samples to be evaluated.
10. Additional IHC Studies with Human Tissues
The studies presented here have been described in detail because they were all performed in the same lab using the same antiserum, staining process and image analysis. In addition, these studies employed the same type of Standard Curve that allowed
semi-quantitation of PAH-DNA adduct values, and therefore the data from different studies could be compared (
Table 1). However, similar types of DNA adduct
semi-quantitation have been reported for other human tissues during investigations of a variety of carcinogens. Additional types of DNA damage evaluated in human tissues by IHC with image analysis include DNA adducts induced by: 4-aminobiphenyl [
43,
44]; 2-amino-1-methyl-6-phenylimidazole[4,5-b]-pyridine (PhIP) [
45]; malondialdehyde [
46]; and endogenous oxidative stress [
47]. PAH-DNA adducts have been detected by IHC in human exfoliated oral cells and urothelial cells [
48,
49] in studies comparing PAH-DNA adducts in smokers and non-smokers. The smokers were recruited from the New York City area [
49] and from Rome, Italy [
48], and both studies showed that smokers had higher levels of PAH-DNA adducts in exfoliated cells than non-smokers. Taken together, all the IHC studies support the ubiquitous nature of DNA adduct formation in human tissues, as PhIP-DNA adducts and 2-aminobiphenyl-DNA adducts were found in normal human breast [
43,
45], 1,
N6-ethenodeoxyadenosine was found in diseased human liver [
47], and several types of adducts were found in exfoliated oral and urothelial cells [
44,
46,
48]. Information regarding DNA adduct distribution in human tissues and cells elucidates human exposure and may inform decisions related to prevention and management of cancer risk.
11. Conclusions and Future Perspectives
Several important conclusions can be drawn from these studies, the data for which are summarized in
Table 1.
First: Environmental and dietary PAH exposures result in PAH-DNA adduct formation simultaneously in many tissues of the body.
Second: PAH-DNA adducts in human tissues are concentrated in organ-specific cell types, a phenomenon which results in higher DNA adduct values determined by IHC/ACIS compared to conventional immunoassay analysis where DNA is extracted from all the cells of a tissue.
Third: These studies confirm a broad inter-individual range (10- to 20-fold) for levels of PAH-DNA adducts in a single tissue type from a single cohort.
Fourth: IHC/ACIS of archived human tissues may be an attractive approach for PAH-DNA adduct evaluation as paraffin blocks are readily available and the current studies show these adducts to be stable in paraffin for at least 20 years.
Fifth: Whereas the assay is only
semi-quantitative, the standard curve stained concurrently with the tissue sections allows comparison of tissues stained at different times, suggesting a consistency in the method that reveals real differences between tissues (
Table 1). For example, it is possible that the high PAH-DNA levels in prostate indicate high levels of exposure in the population studied, but it is also possible that the prostate is particularly susceptible to this type of DNA damage.
Evaluation of the biologically effective dose for PAH exposure, through detection of PAH-DNA adducts, is an important step forward in our understanding of the connection between chemical exposure and cancer development, since cellular events are necessarily more relevant to disease outcome than measurement of PAH levels in the ambient environment or diet. Quantifying PAH exposures by monitoring intake is informative, but does not account for interindividual metabolic variations, which we and others have shown to be substantial. Nor does it reveal tissue-specific differences, which are important since it is evident that not all tissues are targets for tumor formation. In fact, in esophagus, cervix and vulva, observations that PAH-DNA adducts concentrate in the epithelial basal cell layer are consistent with the susceptibility of these cells to tumor induction.
The ability to assess biomarkers of exposure in archived whole tissue will allow for: generation of large quantities of data; new information on cellular processing of DNA damage; and comparison of PAH-DNA information with additional methodologies to elucidate mechanism. Availability of new specialized techniques, such as laser capture microdissection, may provide additional opportunities to collect and evaluate very specific portions of whole tissue, or individual cells, in which PAH-DNA damage can potentially be related to other molecular markers such as gene expression, micro-RNAs or epigenetics.
Acknowledgments
This work was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, NIH. We wish to thank all of our colleagues who contributed to these studies, including: Hilde E. Van Gijssel, Philip R. Taylor, Sanford M. Dawsey, Philip E. Castle, Francis L. Martin, David M. DeMarini, Radim J. Sram, David K. Manchester, Paul Sirajuddin, Ofelia A. Olivero, Rao L. Divi, Laura J. Schild-Hay, Mark J. Roth, and Steven Hewitt.
References
- Gyorffy, E; Anna, L; Kovacs, K; Rudnai, P; Schoket, B. Correlation between biomarkers of human exposure to genotoxins with focus on carcinogen-DNA adducts. Mutagenesis 2008, 23, 1–18. [Google Scholar]
- Phillips, DH; Arlt, VM. Genotoxicity: Damage to DNA and its consequences. EXS 2009, 99, 87–110. [Google Scholar]
- Poirier, MC. Chemical-induced DNA damage and human cancer risk. Nat. Rev. Cancer 2004, 4, 630–637. [Google Scholar]
- Angerer, J; Ewers, U; Wilhelm, M. Human biomonitoring: State of the art. Int. J. Hyg. Environ. Health 2007, 210, 201–228. [Google Scholar]
- Gallo, V; Khan, A; Gonzales, C; Phillips, DH; Schoket, B; Gyorffy, E; Anna, L; Kovacs, K; Moller, P; Loft, S; et al. Validation of biomarkers for the study of environmental carcinogens: A review. Biomarkers 2008, 13, 505–534. [Google Scholar]
- Poirier, MC; Santella, RM; Weston, A. Carcinogen macromolecular adducts and their measurement. Carcinogenesis 2000, 21, 353–359. [Google Scholar]
- Poirier, MC; Beland, FA. DNA adduct measurements and tumor incidence during chronic carcinogen exposure in animal models: Implications for DNA adduct-based human cancer risk assessment. Chem. Res. Toxicol 1992, 5, 749–755. [Google Scholar]
- Swenberg, JA; Fryar-Tita, E; Jeong, YC; Boysen, G; Starr, T; Walker, VE; Albertini, RJ. Biomarkers in toxicology and risk assessment: Informing critical dose-response relationships. Chem. Res. Toxicol 2008, 21, 253–265. [Google Scholar]
- Kyrtopoulos, SA. Biomarkers in environmental carcinogenesis research: Strivisng for a new momentum. Toxicol. Lett 2006, 162, 3–15. [Google Scholar]
- Veglia, F; Matullo, G; Vineis, P. Bulky DNA adducts and risk of cancer: A meta-analysis. Cancer Epidemiol. Biomarkers Prev 2003, 12, 157–160. [Google Scholar]
- Pratt, MM; Reddy, AP; Hendricks, JD; Pereira, C; Kensler, TW; Bailey, GS. The importance of carcinogen dose in chemoprevention studies: Quantitative interrelationships between, dibenzo[a,l]pyrene dose, chlorophyllin dose, target organ DNA adduct biomarkers and final tumor outcome. Carcinogenesis 2007, 28, 611–624. [Google Scholar]
- Simonich, MT; Egner, PA; Roebuck, BD; Orner, GA; Jubert, C; Pereira, C; Groopman, JD; Kensler, TW; Dashwood, RH; Williams, DE; et al. Natural chlorophyll inhibits aflatoxin B1-induced multi-organ carcinogenesis in the rat. Carcinogenesis 2007, 28, 1294–1302. [Google Scholar]
- Stoner, GD; Wang, LS; Casto, BC. Laboratory and clinical studies of cancer chemoprevention by antioxidants in berries. Carcinogenesis 2008, 29, 1665–1674. [Google Scholar]
- Phillips, DH. Fifty years of benzo(a)pyrene. Nature 1983, 303, 468–472. [Google Scholar]
- Yuspa, SH; Poirier, MC. Chemical carcinogenesis: From animal models to molecular models in one decade. Adv. Cancer Res 1988, 50, 25–70. [Google Scholar]
- Poirier, MC. Antibodies to carcinogen-DNA adducts. J. Natl. Cancer Inst 1981, 67, 515–519. [Google Scholar]
- Poirier, MC; Santella, R; Weinstein, IB; Grunberger, D; Yuspa, SH. Quantitation of benzo(a)pyrene-deoxyguanosine adducts by radioimmunoassay. Cancer Res 1980, 40, 412–416. [Google Scholar]
- Weston, A; Manchester, DK; Poirier, MC; Choi, JS; Trivers, GE; Mann, DL; Harris, CC. Derivative fluorescence spectral analysis of polycyclic aromatic hydrocarbon-DNA adducts in human placenta. Chem. Res. Toxicol 1989, 2, 104–108. [Google Scholar]
- IARC. Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures. IARC Monogr. Ser 2010, 92, 1–853.
- Divi, RL; Beland, FA; Fu, PP; Von Tungeln, LS; Schoket, B; Camara, JE; Ghei, M; Rothman, N; Sinha, R; Poirier, MC. Highly sensitive chemiluminescence immunoassay for benzo[a]pyrene-DNA adducts: Validation by comparison with other methods, and use in human biomonitoring. Carcinogenesis 2002, 23, 2043–2049. [Google Scholar]
- Liou, SH; Jacobson-Kram, D; Poirier, MC; Nguyen, D; Strickland, PT; Tockman, MS. Biological monitoring of fire fighters: Sister chromatid exchange and polycyclic aromatic hydrocarbon-DNA adducts in peripheral blood cells. Cancer Res 1989, 49, 4929–4935. [Google Scholar]
- Rothman, N; Poirier, MC; Baser, ME; Hansen, JA; Gentile, C; Bowman, ED; Strickland, PT. Formation of polycyclic aromatic hydrocarbon-DNA adducts in peripheral white blood cells during consumption of charcoal- broiled beef. Carcinogenesis 1990, 11, 1241–1243. [Google Scholar]
- Kang, DH; Rothman, N; Poirier, MC; Greenberg, A; Hsu, CH; Schwartz, BS; Baser, ME; Groopman, JD; Weston, A; Strickland, PT. Interindividual differences in the concentration of 1-hydroxypyrene-glucuronide in urine and polycyclic aromatic hydrocarbon-DNA adducts in peripheral white blood cells after charbroiled beef consumption. Carcinogenesis 1995, 16, 1079–1085. [Google Scholar]
- Rothman, N; Correa-Villasenor, A; Ford, DP; Poirier, MC; Haas, R; Hansen, JA; O’Toole, T; Strickland, PT. Contribution of occupation and diet to white blood cell polycyclic aromatic hydrocarbon-DNA adducts in wildland firefighters. Cancer Epidemiol., Biomarkers Prev 1993, 2, 341–348. [Google Scholar]
- Rothman, N; Poirier, MC; Haas, RA; Correa-Villasenor, A; Ford, P; Hansen, JA; O’Toole, T; Strickland, PT. Association of PAH-DNA adducts in peripheral white blood cells with dietary exposure to polyaromatic hydrocarbons. Environ. Health Perspect 1993, 99, 265–267. [Google Scholar]
- Jacobson-Kram, D; Albertini, RJ; Branda, RF; Falta, MT; Iype, PT; Kolodner, K; Liou, SH; McDiarmid, MA; Morris, M; Nicklas, JA; et al. Measurement of chromosomal aberrations, sister chromatid exchange hprt mutations and DNA adducts in peripheral lymphocytes of human populations at increased risk for cancer. Environ. Health Perspect 1993, 101, 121–125. [Google Scholar]
- Poirier, MC; Weston, A; Schoket, B; Shamkhani, H; Pan, CF; McDiarmid, MA; Scott, BG; Deeter, DP; Heller, JM; Jacobson-Kram, D; et al. Biomonitoring of United States Army soldiers serving in Kuwait in 1991. Cancer Epidemiol. Biomarkers Prev 1998, 7, 545–551. [Google Scholar]
- Garcia-Suastegui, WA; Huerta-Chagoya, A; Carrasco-Colın, KL; Pratt, MM; John, K; Petrosyan, P; Rubio, J; Poirier, MC; Gonsebatt, ME. Seasonal variations in the levels of PAH–DNA adducts in young adults living in Mexico City. Mutagenesis 2011, 26, 385–391. [Google Scholar]
- Gunter, MJ; Divi, RL; Kulldorff, M; Vermeulen, R; Haverkos, KJ; Kuo, MM; Strickland, P; Poirier, MC; Rothman, N; Sinha, R. Leukocyte polycyclic aromatic hydrocarbon-DNA adduct formation and colorectal adenoma. Carcinogenesis 2007, 28, 1426–1429. [Google Scholar]
- Sinha, R; Peters, U; Cross, AJ; Kulldorff, M; Weissfeld, JL; Pinsky, PF; Rothman, N; Hayes, RB. Meat, meat cooking methods and preservation, and risk for colorectal adenoma. Cancer Res 2005, 65, 8034–8041. [Google Scholar]
- Van Gijssel, HE; Divi, RL; Olivero, OA; Roth, MJ; Wang, GQ; Dawsey, SM; Albert, PS; Qiao, YL; Taylor, PR; Dong, ZW; et al. Semi-quantitation of polycyclic aromatic hydrocarbon-DNA adducts in human esophagus by immunohistochemistry and the automated cellular imaging system. Cancer Epidemiol. Biomarkers Prev 2002, 11, 1622–1629. [Google Scholar]
- Messersmith, W; Oppenheimer, D; Peralba, J; Sebastiani, V; Amador, M; Jimeno, A; Embuscado, E; Hidalgo, M; Iacobuzio-Donahue, C. Assessment of Epidermal Growth Factor Receptor (EGFR) signaling in paired colorectal cancer and normal colon tissue samples using computer-aided immunohistochemical analysis. Cancer Biol. Ther 2005, 4, 1381–1386. [Google Scholar]
- Minot, DM; Kipp, BR; Root, RM; Meyer, RG; Reynolds, CA; Nassar, A; Henry, MR; Clayton, AC. Automated cellular imaging system III for assessing HER2 status in breast cancer specimens: Development of a standardized scoring method that correlates with FISH. Am. J. Clin. Pathol 2009, 132, 133–138. [Google Scholar]
- John, K; Ragavan, N; Pratt, MM; Singh, PB; Al-Buheissi, S; Matanhelia, SS; Phillips, DH; Poirier, MC; Martin, FL. Quantification of phase I/II metabolizing enzyme gene expression and polycyclic aromatic hydrocarbon-DNA adduct levels in human prostate. Prostate 2009, 69, 505–519. [Google Scholar]
- Roth, MJ; Strickland, KL; Wang, GQ; Rothman, N; Greenberg, A; Dawsey, SM. High levels of carcinogenic polycyclic aromatic hydrocarbons present within food from Linxian, China may contribute to that region’s high incidence of oesophageal cancer. Eur. J. Cancer 1998, 34, 757–758. [Google Scholar]
- Van Gijssel, HE; Schild, LJ; Watt, DL; Roth, MJ; Wang, GQ; Dawsey, SM; Albert, PS; Qiao, YL; Taylor, PR; et al. Polycyclic aromatic hydrocarbon-DNA adducts determined by semi-quantitative immunohistochemistry in human esophageal biopsies taken in 1985. Mutat. Res 2004, 547, 55–62. [Google Scholar]
- Castle, PE; Wacholder, S; Lorincz, AT; Scott, DR; Sherman, ME; Glass, AG; Rush, BB; Schussler, JE; Schiffman, M. A prospective study of high-grade cervical neoplasia risk among human papillomavirus-infected women. J. Natl. Cancer Inst 2002, 94, 1406–1414. [Google Scholar]
- Pratt, MM; Sirajuddin, P; Poirier, MC; Schiffman, M; Glass, AG; Scott, DR; Rush, BB; Olivero, OA; Castle, PE. Polycyclic aromatic hydrocarbon-DNA adducts in cervix of women infected with carcinogenic human papillomavirus types: An immunohistochemistry study. Mutat. Res 2007, 624, 114–123. [Google Scholar]
- Sram, RJ; Benes, I; Binkova, B; Dejmek, J; Horstman, D; Kotesovec, F; Otto, D; Perreault, SD; Rubes, J; Selevan, SG; et al. Teplice Program: Impact of air pollution on human health. Environ. Health Perspect 1996, 104, 699–714. [Google Scholar]
- Sram, RJ; Binkova, B; Rossner, P; Rubes, J; Topinka, J; Dejmek, J. Adverse reproductive outcomes from exposure to environmental mutagens. Mutat. Res 1999, 428, 203–215. [Google Scholar]
- Pratt, MM; King, LC; Adams, LD; John, K; Sirajuddin, P; Olivero, OA; Manchester, DK; Sram, RJ; DeMarini, DM; Poirier, MC. Assessment of multiple types of DNA damage in human placentas from smoking and nonsmoking women in the Czech Republic. Environ. Mol. Mutagen 2011, 52, 58–68. [Google Scholar]
- Hewitt, SM. Design, construction, and use of tissue microarrays. Methods Mol. Biol 2004, 264, 61–72. [Google Scholar]
- Faraglia, B; Chen, SY; Gammon, MD; Zhang, Y; Teitelbaum, SL; Neugut, AI; Ahsan, H; Garbowski, GC; Hibshoosh, H; Lin, D; et al. Evaluation of 4-aminobiphenyl-DNA adducts in human breast cancer: The influence of tobacco smoke. Carcinogenesis 2003, 24, 719–725. [Google Scholar]
- Hsu, TM; Zhang, YJ; Santella, RM. Immunoperoxidase quantitation of 4-aminobiphenyl- and polycyclic aromatic hydrocarbon-DNA adducts in exfoliated oral and urothelial cells of smokers and nonsmokers. Cancer Epidemiol., Biomarkers & Prev 1997, 6, 193–199. [Google Scholar]
- Zhu, J; Chang, P; Bondy, ML; Sahin, AA; Singletary, SE; Takahashi, S; Shirai, T; Li, D. Detection of 2-amino-1-methyl-6-phenylimidazo[4,5-b]-pyridine-DNA adducts in normal breast tissues and risk of breast cancer. Cancer Epidemiol., Biomarkers & Prev 2003, 12, 830–837. [Google Scholar]
- Zhang, Y; Chen, SY; Hsu, T; Santella, RM. Immunohistochemical detection of malondialdehyde-DNA adducts in human oral mucosa cells. Carcinogenesis 2002, 23, 207–211. [Google Scholar]
- Frank, A; Seitz, HK; Bartsch, H; Frank, N; Nair, J. Immunohistochemical detection of 1,N6-ethenodeoxyadenosine in nuclei of human liver affected by diseases predisposing to hepato-carcinogenesis. Carcinogenesis 2004, 25, 1027–1031. [Google Scholar]
- Romano, G; Sgambato, A; Boninsegna, A; Flamini, G; Curigliano, G; Yang, Q; La Gioia, V; Signorelli, C; Ferro, A; Capelli, G; et al. Evaluation of polycyclic aromatic hydrocarbon-DNA adducts in exfoliated oral cells by an immunohistochemical assay. Cancer Epidemiol. Biomarkers Prev 1999, 8, 91–96. [Google Scholar]
- Zhou, Q; Talaska, G; Jaeger, M; Bhatnagar, VK; Hayes, RB; Zenzer, TV; Kashyap, SK; Lakshmi, VM; Kashyap, R; Dosemeci, M; et al. Benzidine-DNA adduct levels in human peripheral white blood cells significantly correlate with levels in exfoliated urothelial cells. Mutat. Res 1997, 393, 199–205. [Google Scholar]
Abbreviations
| ACIS | Automated Cellular Imaging System |
| BP | benzo[a]pyrene |
| BPDE | r7, t8-dihydroxy-t-9, 10-epoxy-7, 8, 9, 10-tetrahydrobenzo[a]pyrene |
| BPdG | r7, t8, t9-trihydroxy-c-10-(N2deoxyguanosyl)-7, 8, 9, 10-tetrahydro-benzo[a]pyrene |
| CIA | chemiluminescence immunoassay |
| CT | cytotrophoblast |
| HPV | Human Papillomavirus |
| IHC | immunohistochemistry |
| LOD | limit of detection |
| OD | optical density, or mean color intensity reported by the ACIS |
| PAH | polycyclic aromatic hydrocarbon |
| PhIP | 2-amino-1-methyl-6-phenylimidazole[4,5-b]-pyridine |
| PZ | peripheral zone of the human prostate |
| ST | syncytiotrophoblast |
| TZ | transition zone of the human prostate |
| VIN | Vulvar Intraepithelial Neoplasia |
© 2011 by the authors; licensee MDPI, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).