Pigmented Fungiform Papillae (PFP) of the Tongue: A Systematic Review of Current Aetiopathogenesis and Pathophysiology
Abstract
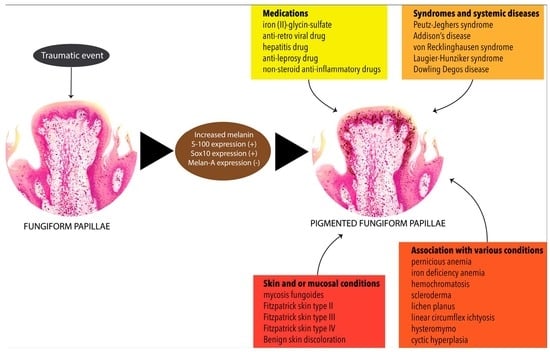
:1. Introduction
2. Materials and Methods
3. Results
3.1. Cumulative Data
3.2. Clinical Appearance of PFP
3.3. Associated Dermatological and Other Systemic Manifestations
| Gender | Age | Ethnicity | Location of Affected Papillae | Clinical Appearance | Dermoscopy | Type | Ref. |
|---|---|---|---|---|---|---|---|
| Female | 7 | Ethiopian | Antero-dorsal | Multiple brown pigmented spots | RP | Type 2 | [63] |
| Female | 8 | African | Antero-lateral | Multiple dark pinhead papules | RP | Type 2 | [57] |
| Female | 9 | Japanese | Anterial and dorsal | Multiple pigmentation | CS | Type 1 | [64] |
| Female | 9 | NR | Lateral | Multiple pigmentation | NR | Type 1 | [43] |
| Female | 10 | Indian | Dorsal and lateral | Multiple sharply bordered macules | NR | Type 2 | [55] |
| Female | 12 | Moroccan | Anterial | Multiple hyperpigmented papillae in a diffuse and symmetrical pattern | RP | Type 1 | [46] |
| Female | 12 | African | Anterial, lateral, and dorsal | Multiple hyperpigmented papillae presenting as dark patches | RP | Type 2 | [36] |
| Female | 12 | NR | Anterial and dorsal | Multiple discrete tan-brown pinhead papules | RP | Type 2 | [58] |
| Female | 12 | NR | Dorsal and lateral | Brown pigmentations | RP CS | Type 1 | [47] |
| Female | 12 | Asian | Anterial | Tiny pigmented macules | CS | Type 2 | [65] |
| Female | 13 | South Asian | Anterial | Light to dark brown pigmentation, round or polygonal in shape, and circumscribed | CS | Type 1 | [66] |
| Female | 13 | Mexican | NR | Multiple hyperchromic macules, light brown, mottled, 1 mm in diameter | RP | Type 2 | [48] |
| Female | 15 | NR | Anterial, lateral, and dorsal | Asymptomatic and multiple brown pigmentations | CS RP | Type 1 Type 2 | [49] |
| Female | 15 | NR | Dorsal | Multiple brown macules | NR | Type 2 | [31] |
| Female | 18 | North African | Anterial | Multiple small erythematous and hyperpigmented papules | RP | Type 1 | [50] |
| Female | 18 | Black | Dorsal | Bluish-black to black macular hyperpigmentation measuring 30–70 mm | NR | Type 1 | [33] |
| Female | 18 | NR | Dorsal | Dark spots | NR | NCP | [51] |
| Female | 20 | NR | Antero-lateral and dorsal | Multiple brown macules | RP | Type 2 | [67] |
| Female | 20 | Moroccan | Dorsal | NR | NR | Type 2 | [68] |
| Female | 21 | NR | Antero-lateral | Irregularly distributed pigmentation | RP | Type 2 | [42] |
| Female | 23 | NR | Antero-lateral | Multiple hyperpigmented papillae in a diffuse pattern | NR | Type 2 | [56] |
| Female | 24 | Hispanic | Dorsal and lateral | Diffuse punctate pigmentation in a symmetrical pattern, and others grouped in a mottled pattern | RP | Type 1 | [69] |
| Female | 25 | Saudi | Dorsal | Diffuse tan, brown, patches with prominent dark papillae | NR | Type 2 | [52] |
| Female | 25 | Korean | Antero-lateral | Multiple dark brownish macules | NR | Type 1 | [70] |
| Female | 33 | Korean | Antero-lateral | Multiple dark brownish macules | NR | Type 1 | |
| Female | 26 | Black | Antero-lateral | Small reddish-brown pigmented lesions | NR | Type 1 | [71] |
| Female | 26 | Indian | Dorsal | Multiple tiny brown macules | NR | Type 1 | [37] |
| Female | 27 | Italian | Antero-lateral | Multiple blue-grey pigmentation, diffuse with a symmetrical pattern | RP | Type 2 | [72] |
| Female | 28 | Haitian | Antero-lateral | Multiple dark brown macules | NR | Type 2 | [73] |
| Female | 28 | NR | Dorsal | Multiple discrete tan-brown pin-head papules | RP | Type 2 | [53] |
| Female | 29 | NR | Dorsal | Hyperpigmentation, diffuse with a symmetrical pattern | NR | Type 2 | [61] |
| Female | 30 | NR | Dorsal | Hyperpigmented macules | RP | Type 2 | [30] |
| Female | 30 | Black | Anterial | Multiple hyperpigmented papillae | RP | Type 2 | [74] |
| Female | 32 | Caucasian | Dorsal | 6 mm oval area with brown pigmentation | NR | Type 2 | [75] |
| Female | 35 | Japanese | Antero-lateral | Hyperpigmented papillae | RP | Type 1 | [44] |
| Female | 35 | African | Dorsal | Groups of 15 to 20 papillae with a mottled appearance | NR | Type 2 | [76] |
| Female | 43 | South American | Dorsal | Diffuse and symmetrical pattern of macules | NR | Type 1 | |
| Female | 40 | Black | Anterial | Multiple hyperpigmented papillae | RP | Type 2 | [39] |
| Female | 44 | Black | Anterial | Multiple hyperpigmented papillae | RP | Type 2 | |
| Female | 44 | African | Antero-lateral | NR | NR | Type 2 | [77] |
| Female | 45 | Hispanic | Lateral | Multiple dark-brown pigmented papules | NR | Type 1 | [78] |
| Female | NR * | NR | Dorsal and lateral | Multiple hyperpigmented papillae and patches | CS | Type 2 | [38] |
| Male | 8 | Latin America | Anterial and dorsal | Multiple asymptomatic and sharply demarcated hyperpigmented pinhead papules | CS | Type 2 | [59] |
| Male | 11 | Brazilian | Anterial and lateral | Multiple tiny brown macules | NR | Type 1 | [62] |
| Male | 12 | NR | Dorsal and lateral | Multiple pigmented papule in a symmetrical pattern | NR | Type 1 | [41] |
| Male | 17 | Korean | Anterial | Well-demarcated small, black, clustered hyperpigmented papules | NR | Type 1 | [79] |
| Male | 26 | Taiwanese | Antero-lateral | Multiple tiny brown macules | CS | Type 2 | [80] |
| Male | 28 | NR | Anterial | Multiple dark-brown macules and dome shaped papules | CS | Type 1 | [34] |
| Male | 28 | Hispanic | Latero-distal | Multiple dark-brown pigmented papules | RP | NCP | [35] |
| Male | 36 | Italian | Dorsal and lateral | Multiple brown papillae | CS | Type 2 | [81] |
| Male | 42 | Japanese | Lateral | Black pigmented papillae | NR | Type 1 | [40] |
| Male | 65 | Vietnamese | Dorsal and lateral | NR | NR | Type 2 | [82] |
| Female | 21 | Javanese | Dorsal and lateral | Multiple brownish-black, diffuse, and asymptomatic macules | CS | Type 2 | [60] |
| Male | 22 | Javanese | Dorsal | Multiple macules, brownish-black and sharing a clear border | CS | Type 2 | |
| Female Female Female Female Female Female Female Female Female Female Female Female Male Male Male | 18 18 22 26 27 29 31 36 39 40 48 51 8 24 52 | Mixed ethnicity | Not reported individually in each patient | patient | Not reported individually in each patient | Type 2 Type 2 Type 2 Type 2 Type 2 Type 2 Type 2 Type 1 Type 2 Type 2 Type 2 Type 2 Type 1 Type 2 Type 2 | [54] |
3.4. Complete Blood Cell Count and Blood Chemistry Findings
3.5. Liver and Kidney Function Tests
3.6. Infection Markers, Endocrine, Auto-Immune, Ophthalmological, and Neurological Findings
3.7. Additional Findings
3.8. Histopathology
3.9. Immunohistochemistry
3.10. Other Features
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ko, E.; Panchal, N. Pigmented Lesions. Dermatol. Clin. 2020, 38, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Feller, L.; Khammissa, R.A.G.; Lemmer, J. Oral Mucosal Melanosis. In Melanin; InTech: Rijeka, Croatia, 2017; pp. 7–26. [Google Scholar]
- Verma, B.; Bahra, A.; Ajmal, A.K.M.; Sen, S. Laugier–Hunziker Syndrome in a Young Female. Indian Dermatol. Online J. 2017, 8, 148–150. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.S.; Oh, C.H.; Coffin, S.E.; Yan, A.C. Addisonian pigmentation of the oral mucosa. Cutis 2005, 76, 97–99. [Google Scholar] [PubMed]
- Kothiwale, S. An Oral Clinician’s Perspective towards Peutz Jegher’s Syndrome A Case Report. J. Probiotics Health 2015, 3, 128. [Google Scholar] [CrossRef]
- Agrawal Sharma, Y.K.; Deo, K.S.; Ranpariya, R.H.; Singh, P. Mucocutaneous hyperpigmentation as presentation of vitamin B12 deficiency: A case report and brief review. Pigment. Int. 2019, 6, 37–42. [Google Scholar]
- Song, X.; Shen, Y.; Zhou, Y.; Lou, Q.; Han, L.; Ho, J.K.; Ren, Y. General hyperpigmentation induced by Grave’s disease. Medicine 2018, 97, e13279. [Google Scholar] [CrossRef]
- Chandran, R.; Feller, L.; Lemmer, J.; Khammissa, R.A.G. HIV-Associated Oral Mucosal Melanin Hyperpigmentation: A Clinical Study in a South African Population Sample. AIDS Res. Treat. 2016, 2016, 8389214. [Google Scholar] [CrossRef]
- Lundin, K.; Schmidt, G.; Bonde, C. Amalgam Tattoo Mimicking Mucosal Melanoma: A Diagnostic Dilemma Revisited. Case Rep. Dent. 2013, 2013, 787294. [Google Scholar] [CrossRef]
- Kauzman, A.; Pavone, M.; Blanas, N.; Bradley, G. Pigmented lesions of the oral cavity: Review, differential diagnosis, and case presentations. J. Can. Dent. Assoc. 2004, 70, 682–683. [Google Scholar]
- Hassona, Y.; Sawair, F.; Al-karadsheh, O.; Scully, C. Prevalence and clinical featuRes. of pigmented oral lesions. Int. J. Dermatol. 2016, 55, 1005–1013. [Google Scholar] [CrossRef]
- Ware, R.E.; Aygun, B. Advances in the use of hydroxyurea. Hematology 2009, 2009, 62–69. [Google Scholar] [CrossRef]
- Bloom, M.D.; Gibney, J.M.; Heldermon, C.D. Pigmentation of the tongue with lapatinib treatment in a patient with advanced breast cancer: A case report. Cancer Treat Commun. 2016, 7, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Stringer, L.L.; Laura, Z. Hyperpigmentation of the Tongue. J. Adv. Pract. Oncol. 2014, 5, 71–72. [Google Scholar]
- Kim, H.S.; Park, Y.M.; Kim, H.O.; Lee, J.Y. Tegafur-induced hyperpigmentation of the tongue. J. Dermatol. 2010, 37, 937–938. [Google Scholar] [CrossRef]
- Vasudevan, B. An unusual case of capecitabine hyperpigmentation: Is hyperpigmentation a part of hand-foot syndrome or a separate entity. Indian J. Pharmacol. 2010, 42, 326–328. [Google Scholar] [CrossRef]
- Williams, M.; Hatipoglu, B. Development of Abnormal Pigmentation of the Oral Mucosa Following Adjuvant Radioactive Iodine Treatment. AACE Clin. Case Rep. 2016, 2, e113–e116. [Google Scholar] [CrossRef]
- Mlika, R.B.; Kerkeni, N.; Marrak, H.; Fenniche, S.; Mokhtar, I.; Debbiche, A. Tongue hyperpigmentation during PEG-interferon-alfa/ribavirin therapy in a non-Caucasian patient with chronic hepatitis C: A case report and review of the literature. Int. J. Dermatol. 2013, 52, 631–644. [Google Scholar] [CrossRef]
- Mattoo, K.; Singh, M.; Arora, P. Bilateral Smokers Melanosis—Rare site occurrence in an edentulous patient—A case report. Med. Res. Chron. 2014, 1, 97–101. [Google Scholar]
- Carlos-Bregni, R.; Contreras, E.; Netto, A.C.; Mosqueda-Taylor, A.; Vargas, P.A.; Jorge, J.; León, J.E.; de Almeida, O.P. Oral melanoacanthoma and oral melanotic macule: A report of 8 cases, review of the literature, and immunohistochemical analysis. Med. Oral Patol. Oral Cir. Bucal. 2007, 12, E374–E379. [Google Scholar] [PubMed]
- Sreeja, C.; Ramakrishnan, K.; Vijayalakshmi, D.; Devi, M.; Aesha, I.; Vijayabanu, B. Oral pigmentation: A review. J. Pharm. Bioallied. Sci. 2015, 7, S403–S408. [Google Scholar] [CrossRef]
- Tlholoe, M.M.; Khammissa, R.A.G.; Bouckaert, M.; Altini, M.; Lemmer, J.; Feller, L. Oral Mucosal Melanoma: Some Pathobiological Considerations and an Illustrative Report of a Case. Head Neck Pathol. 2015, 9, 127–134. [Google Scholar] [CrossRef]
- Sudarshan, R.; Vijayabala, G.S.; Samata, Y.; Ravikiran, A. Newer Classification System for Fissured Tongue: An Epidemiological Approach. J. Trop. Med. 2015, 2015, 262079. [Google Scholar] [CrossRef] [PubMed]
- Casu, C.; Giulia Nosotti, M.; Sinesi, A.; Mosaico, G. Hairy tongue, geographic tongue, scrotal tongue and systemic connections: Clinical images and an overview. Dent. Case Rep. 2019, 3, 1–5. [Google Scholar]
- Mangold, A.R.; Torgerson, R.R.; Rogers, R.S. Diseases of the tongue. Clin. Dermatol. 2016, 34, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Pinna, R.; Cocco, F.; Campus, G.; Conti, G.; Milia, E.; Sardella, A.; Cagetti, M.G. Genetic and developmental disorders of the oral mucosa: Epidemiology; molecular mechanisms; diagnostic criteria; management. Periodontol. 2000 2019, 80, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Chaubal, T.V.; Dixit, M.B. Ankyloglossia and its management. J. Indian Soc. Periodontol. 2011, 15, 270–272. [Google Scholar] [CrossRef] [PubMed]
- Alaoui, F.; Souissi, A.; Jendoubi, F.; Mokni, M. Caviar tongue: A lingual physiological variation. Presse Med. 2018, 47, 925–926. [Google Scholar] [CrossRef] [PubMed]
- Marcoval-Caus, J. Response to: «Pigmented Fungiform Papillae of the Tongue in Laugier Disease (or Laugier-Hunziker Syndrome)». Actas Dermo-Sifiliográficas 2013, 104, 174. [Google Scholar] [CrossRef]
- Pinos-León, V.; Granizo-Rubio, J.; Torres-Cruz, M. Enfermedad de Dowling Degos asociada a papilas fungiformes pigmentadas de la lengua: Reporte de un caso. Piel 2015, 30, 15–18. [Google Scholar] [CrossRef]
- Rice, S.M.; Lal, K. Successful Treatment of Pigmented Fungiform Papillae of the Tongue With Q-switched Ruby Laser. Dermatol. Surg. 2022, 48, 368–369. [Google Scholar] [CrossRef]
- Surboyo, M.D.C.; Santosh, A.B.R.; Hariyani, N.; Ernawati, D.S.; Cecilia, P.H. Clinical utility of dermoscopy on diagnosing pigmented papillary fungiform papillae of the tongue: A systematic review. J. Oral Biol. Craniofac. Res. 2021, 11, 618–623. [Google Scholar] [CrossRef]
- Holzwanger, J.M.; Rudolph, R.I.; Heaton, C.L. Pigmented fungiform papillae of the tongue: A common variant of oral pigmentation. Int. J. Dermatol. 1974, 13, 403–408. [Google Scholar] [CrossRef]
- Chamseddin, B.; Vandergriff, T. Pigmented fungiform papillae of the tongue: A clinical and histologic description. Dermatol. Online J. 2019, 25, 11. [Google Scholar] [CrossRef]
- Maxim, E.; de Dtefano, D. Pigmented fungiform papillae of the tongue: An incidental finding in a patient with mycosis fungoides. J. Am. Acad. Dermatol. 2018, 79, AB221. [Google Scholar]
- Docx, M.K.F.; Vandenberghe, P.; Govaert, P. Pigmented fungiform papillae of the tongue (PFPT). Acta Clin. Belg. 2016, 71, 117–118. [Google Scholar] [CrossRef] [PubMed]
- Millington, G.; Shah, S. A case of pigmented fungiform lingual papillae in an Indian woman. J. Eur. Acad. Dermatol. Venereol. 2007, 21, 070209222700054. [Google Scholar] [CrossRef]
- Ross, C.L.; Ring, C.; Yang, S. Pigmented Fungiform Papillae of the Tongue. JAMA Dermatol. 2020, 156, 1249. [Google Scholar] [CrossRef]
- Mukamal, L.V.; Ormiga, P.; Ramos-e-silva, M. Dermoscopy of the pigmented fungiform papillae of the tongue. J. Dermatol. 2012, 39, 397–399. [Google Scholar] [CrossRef]
- Isogai, Z.; Kanzaki, T. Pigmented fungiform papillae of the tongue. J. Am. Acad. Dermatol. 1993, 29, 489–490. [Google Scholar] [CrossRef]
- Sharma, S.S.; Sharma, S.S. Pigmentation of the Fungiform Papillae of the Tongue in a Child Secondary to Iron Deficiency Anaemia: An Uncommon Occurrence and Association. Int. J. Oral Health Dent. 2016, 2, 39–42. [Google Scholar] [CrossRef]
- Radomska, A.; Sławińska, M.; Sobjanek, M. Pigmented fungiform papillae of the tongue. Dermatol. Rev. Przegląd Dermatol. 2021, 108, 191–193. [Google Scholar]
- Belysheva, T.S.; Vishnevskaya, Y.V.; Kletskaya, I.S.; Michenko, A.V.; Volkova, A.S.; Valiev, T.T. Problems of clinical and morphologic diagnosis of orolabial melanosis in children. Russ. J. Pediatric Hematol. Oncol. 2021, 8, 116–122. [Google Scholar] [CrossRef]
- Mizawa, M.; Makino, T.; Furukawa, F.; Torai, R.; Shimizu, T. Efficacy of Q-switched ruby laser treatment for pigmented fungiform papillae of the tongue. J. Dermatol. 2022, 49, e133–e134. [Google Scholar] [CrossRef] [PubMed]
- Samaras, G.; Bikos, D.; Vieira, J.; Hartmann, C.; Charalambides, M.; Hardalupas, Y.; Masen, M.; Cann, P. Measurement of molten chocolate friction under simulated tongue-palate kinematics: Effect of cocoa solids content and aeration. Curr. Res. Food Sci. 2020, 3, 304–313. [Google Scholar] [CrossRef]
- Aqil, N.; Bennani, M.; Nassiri, A.; Meziane, M.; Gallou, J.S.; Mernissi, F.Z. Pigmented fungiform papillae of the tongue: Clinical and dermoscopic features. Our Dermatol. Online 2019, 10, 213–214. [Google Scholar] [CrossRef]
- Kavak, A.; Topkarcı, Z.; Gür, E.E.; Erdoğan, B.; Yemişçi, A. Pigmented fungiform papillae of the tongue: Co-existence of two patterns in the same patient and associated dental pigmentation. Turkderm Turk. Arch. Dermatol. Venereol. 2021, 55, 45–47. [Google Scholar] [CrossRef]
- Castellanos, M.A.; Echeverria, A.E.B.; Rosas, G.M.A. Dermatoscopía en la pigmentación de papilas fungiformes de la lengua. Dermatol. Cosmet. Med. Quir. 2018, 16, 206–207. [Google Scholar]
- Özaslan, M. Barwnikowe brodawki grzybowate języka. Dermatol. Rev. 2021, 108, 550–553. [Google Scholar] [CrossRef]
- Cinotti, E.; Labeille, B.; Cambazard, F.; Perrot, J.L. Dermoscopy and reflectance confocal microscopy examination of pigmented fungiform papillae of the tongue. Ann. Dermatol. Venereol. 2017, 144, 323–325. [Google Scholar] [CrossRef]
- Ogrum, A.; Takci, Z.; Yildiz Seckin, H. Pigmented Fungiform Papillae: Case Report and Review of the Literature. Turk. Klin. J. Dermatol. 2018, 28, 32–34. [Google Scholar] [CrossRef]
- Alzahrani, N.; Alharithy, R. Pigmented fungiform papillae of the tongue in a Saudi woman. J. Dermatol. Dermatol. Surg. 2018, 22, 39. [Google Scholar]
- Sil, A.; Panigrahi, A.; Bhanja, D. Rose petal appearance: The dermoscopic finding in pigmented fungiform papillae of the tongue. Indian Dermatol. Online J. 2021, 12, 490–491. [Google Scholar] [CrossRef]
- Garzón-Rivas, V.; Garzón-Aldás, E. Papilas Fungiformes Pigmentadas de la Lengua. Características Clínicas, Histológicas y Dermatoscópicas de una Serie de Casos Ecuatorianos. Int. J. Odontostomatol. 2019, 13, 446–451. [Google Scholar] [CrossRef]
- Werchniak, A.E.; Storm, C.A.; Dinulos, J.G.H. Hyperkeratotic Plaques on the Palms and Soles A Red-Brown Plaque on the Nape Hyperpigmented Patches on the Tongue of a Young Girl. Arch. Dermatol. 2009, 140, 1275–1280. [Google Scholar]
- Gopinath, H.; Upadya, G.M. Pigmented fungiform papillae in mother and daughter. Indian J. Dermatol. Venereol. Leprol. 2017, 85, 510–511. [Google Scholar] [CrossRef]
- Neri, I.; Evangelista, V.; Veronesi, G.; Guglielmo, A.; Chessa, M.A. Oral pigmentation in children: A diagnostic challenge. Arch. Dis. Child. 2021, 106, 895. [Google Scholar] [CrossRef]
- Sil, A.; Panigrahi, A.; Bhanja, D.B. Tongue Discoloration in a Young Girl. J. Pediatr. 2020, 219, 276–277. [Google Scholar] [CrossRef]
- Piquero-Casals, J.; Morgado-Carrasco, D. Pigmented Fungiform Lingual Papillae. J. Pediatr. 2020, 224, 177–178. [Google Scholar] [CrossRef]
- Surboyo, M.D.C.; Ernawati, D.S.; Parmadiati, A.E.; Marsetyo, R.I. Pigmented fungiform papillae of the tongue and lingual fimbriae as single presentation in adult: A case report and literature review. Eur. J. Dent. 2020, 14, 702–706. [Google Scholar] [CrossRef]
- Al-Fagaan, F.; Joseph, B. A case of pigmented fungiform papillae of the tongue in a Middle Eastern Woman. Med. Princ. Pract. 2014, 23, 167–169. [Google Scholar] [CrossRef]
- Romiti, R.; de Medeiros, L.M. Pigmented Fungiform Papillae of The Tongue. Pediatr. Dermatol. 2010, 27, 398–399. [Google Scholar] [CrossRef]
- García Martínez, F.J.; López Martín, I.; Segurado Rodríguez, M.A. Pigmentación de las papilas fungiformes linguales. Pediatría Atención Primaria 2015, 17, e205–e207. [Google Scholar] [CrossRef]
- Sugiyama, Y.; Hayashi, K.; Takayama, T. Pigmented fungiform papillae of the tongue in a Japanese child. Clin. Case Rep. 2020, 8, 1104–1106. [Google Scholar] [CrossRef]
- Lin, Y.T.; Chou, C.L. Pigmented macule on the tongue of a 12-year-old girl. J. Am. Acad Dermatol. 2013, 69, e229–e230. [Google Scholar] [CrossRef]
- Collgros, H.; Iglesias-Sancho, M.; Galvany, L. Dermoscopy of pigmented fungiform papillae of the tongue in an Indian girl. Australas. J. Dermatol. 2015, 56, e81–e82. [Google Scholar] [CrossRef]
- Robles-Mendez, J.C.; Ayala-Cortes, A.S.; Villarreal-Martinez, A.; Ocampo-Candiani, J. Dermoscopy of the pigmented fungiform papillae of the tongue. J. Dermatol. 2017, 76, s40–s42. [Google Scholar] [CrossRef]
- el Anzi, O. Pigmented fungiform papillae of the tongue: Moroccan case. Our Dermatol. Online 2019, 10, 215–216. [Google Scholar] [CrossRef]
- Anco-Gallegos, K.; San, M.; Serrano-Guillén, G. Pigmentacion de las papilas fungiformes de la lengua. Dermatol. Perú 2016, 26, 227–230. [Google Scholar]
- Choi, S.J.; Rhonark, K.; Yang, J.M.; Lee, E.S. Two Cases of Pigmented Fungiform Papillae of the Tongue. Ann. Dermatol. 2002, 14, 216–219. [Google Scholar] [CrossRef]
- Koplon, B.S. Prominent Pigmented Papillae of the Tongue. Arch. Dermatol. 1967, 95, 394–396. [Google Scholar] [CrossRef]
- Ghigliotti, G.; Chinazzo, C.; Parodi, A.; Rongioletti, F. Pigmented fungiform papillae of the tongue: The first case in an Italian woman. Clin. Exp. Dermatol. 2017, 42, 206–208. [Google Scholar] [CrossRef] [PubMed]
- Pehoushek, J.F.; Norton, S.A. Black taste buds. Arch. Fam. Med. 2000, 9, 219–220. [Google Scholar] [CrossRef]
- Pinos-León, V. Dermoscopic FeatuRes. of Pigmented Fungiform Papillae of the Tongue. Case Res. Lett. 2015, 106, 593–594. [Google Scholar] [CrossRef]
- McCarthy, C.; Holt, D.; Triantafyllou, A. Solitary pigmentation of the tongue: Lentigo simplex or pigmented fungiform papilla? Oral Surg. 2016, 11, 50–54. [Google Scholar] [CrossRef]
- Marcoval, J.; Notario, J.; Martin-Sala, S.; Figueras, I. Pigmentation of the Fungiform Papillae of the Tongue: A Report of 2 Cases. Actas Dermo-Sifiliográficas 2011, 102, 739–740. [Google Scholar] [CrossRef]
- Lake, P.L. Dark Spots on the Tongue. J. Dermatol. Nurses Assoc. 2017, 9, 146–147. [Google Scholar] [CrossRef]
- Adibi, S.; Bouquot, J.E. Papillary tip melanosis (pigmented fungiform lingual papillae). Tex. Dent. J. 2011, 128, 573–574. [Google Scholar]
- Oh, C.K.; Kim, M.B.; Jang, H.S.; Kwon, K.S. A case of pigmented fungiform papillae of the tongue in an Asian male. J. Dermatol. 2000, 27, 350–351. [Google Scholar] [CrossRef]
- Hsiao, Y.H.; Ko, J.H.; Lu, C.F.; Chen, M.J. Dermoscopic findings in pigmented fungiform papillae of the tongue. Eur. J. Dermatol. 2011, 21, 819–820. [Google Scholar] [CrossRef]
- Smogorzewski, J.M.; Armstrong, P.; Young, L. Pigmented fungiform papillae of the tongue in an Indian male. Cutis 2019, 103, E16–E17. [Google Scholar]
- Scarff, C.E.; Marks, R. Pigmented fungiform papillae of the tongue in an Indian male. Australas. J. Dermatol. 2003, 44, 149–151. [Google Scholar] [CrossRef]
- Hirobe, T. Keratinocytes regulate the function of melanocytes. Dermatol. Sin. 2014, 32, 200–204. [Google Scholar] [CrossRef]
- Moreiras, H.; Seabra, M.C.; Barral, D.C. Melanin Transfer in the Epidermis: The Pursuit of Skin Pigmentation Control Mechanisms. Int. J. Mol. Sci. 2021, 22, 4466. [Google Scholar] [CrossRef]
- Attili, S. Dermal melanophages: Does quantitative assessment play a role in the diagnosis of inflammatory skin diseases? Indian J. Dermatopathol. Diagn. Dermatol. 2018, 5, 133–134. [Google Scholar] [CrossRef]
- Steigmann, S. The relationship between physiologic pigmentation of the skin and oral mucosa in Yemenite Jews. Oral Surg. Oral Med. Oral Pathol. 1965, 19, 32–38. [Google Scholar] [CrossRef]
- Hossain, M.R.; Ansary, T.M.; Komine, M.; Ohtsuki, M. Diversified Stimuli-Induced Inflammatory Pathways Cause Skin Pigmentation. Int. J. Mol. Sci. 2021, 22, 3970. [Google Scholar] [CrossRef]
- Mordoh, A.; Casas, G.; Horacio, C.; Werner, L.; Aguas, S.C.; Lanfranchi, H.; Krupitzki, H.; Gandolfo, M. Dermoscopic Evaluation Improves Clinical Diagnosis of Oral Melanotic Macules: A Study in 50 Patients with Oral Pigmented Lesions. Am. J. Oral Med. 2018, 4, 1–9. [Google Scholar] [CrossRef]
- Tan, C.; Liu, Y.; Min, Z.S.; Zhu, W.Y. A clinical analysis of 58 Chinese cases of pigmented fungiform papillae of the tongue. J. Eur. Acad. Dermatol. Venereol. 2012, 28, 242–245. [Google Scholar] [CrossRef]
- Mallagray-Montero Md Moreno-López, L.A.; Cerero-Lapiedra, R.; Castro-Janeiro, M.; Madrigal-Martínez-Pereda, C. Medication related to pigmentation of oral mucosa. Med. Oral Patol. Oral Cir. Bucal. 2022, 27, e230–e237. [Google Scholar] [CrossRef]
- Binmadi, N.O.; Bawazir, M.; Alhindi, N.; Mawardi, H.; Mansour, G.; Alhamed, S.; Alfarabi, S.; Akeel, S.; Almazrooa, S. Medication-Induced Oral Hyperpigmentation: A Systematic Review. Patient Prefer. Adherence 2020, 14, 1961–1968. [Google Scholar] [CrossRef]




| Gender | Ages | Ethnicity | Histopathological Findings | Ref |
|---|---|---|---|---|
| Female | 9 | Japanese |
| [64] |
| Female | 9 | NR |
| [43] |
| Female | 12 | Asian | Melanophages in the submucosa | [65] |
| Female | 18 | Black | Chromatophores in the subepidermal area and around the blood vessels within the fungiform papillae | [33] |
| Female | 18 | NR | Melanophages were found in the lamina propria of the papillae | [51] |
| Female | 20 | NR | Melanophages in the lamina propria | [67] |
| Female | 24 | Hispanic | Melanophages in the lamina propria | [69] |
| Female | 25 | Korean | Melanophages in the upper lamina propria | [70] |
| Female | 33 | Korean | Melanophages in the upper lamina propria | |
| Female | 26 | Black |
| [71] |
| Female | 27 | Italian |
| [72] |
| Female | 28 | Haitian |
| [73] |
| Female | 32 | Caucasian | Melanophages were prominent in the fungiform and adjacent filiform papillae | [75] |
| Female | 35 | Japanese |
| [44] |
| Female | 45 | Hispanic | Melanin deposits in the epithelium | [78] |
| Male | 12 | NR | Melanophages in the subepidermal area | [41] |
| Male | 17 | Korean | Melanophages in the upper lamina propria without significant inflammation | [79] |
| Male | 26 | Taiwanese | Pigmented basal keratinocytes and melanophages in the upper lamina propria | [80] |
| Male | 28 | NR |
| [34] |
| Male | 28 | Hispanic | Melanophages within the lamina propria | [35] |
| Male | 42 | Japanese | Melanophages in the upper lamina propria without significant inflammation | [40] |
| Female Female Female Female Female Female Female Female Female Female Female Female Male Male Male | 18 18 22 26 27 29 31 36 39 40 48 51 8 24 52 | Mixed ethnicity |
| [54] |
| Gender | Ages | Ethnicity | Routine Staining | Special Staining | Immunohistochemical Staining | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|
| Marker | Staining | Marker | Staining | Marker | Staining | ||||
| Female | 9 | NR | Brown pigment in the basal layer of keratinocytes | HE | Iron and hemosiderin not detected | Perl’s | Immuno-reactive for Sox-10 | IHC | [43] |
| Female | 27 | Italian |
| HE | - | - | Immuno-reactive of CD3+ T lymphocytes | IHC | [72] |
| Female | 32 | Caucasian | The stroma supporting the fungiform and adjacent filiform papillae showed prominent melanophages | HE | - | - |
| IHC | [75] |
| Female | 35 | Japanese |
| HE | Melanin was detected | Fontana–Masson | - | - | [44] |
| Iron and hemosiderin not detected | Berlin blue | - | - | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Surboyo, M.D.C.; Samaranayake, L.; Santosh, A.B.R.; Ayuningtyas, N.F.; Wati, S.M.; Rahayu, R.P.; Urbina, F.; Kuntari, W.L.; Syahnia, S.J.M.R.; Puspasari, K.; et al. Pigmented Fungiform Papillae (PFP) of the Tongue: A Systematic Review of Current Aetiopathogenesis and Pathophysiology. Pathophysiology 2022, 29, 555-569. https://doi.org/10.3390/pathophysiology29030043
Surboyo MDC, Samaranayake L, Santosh ABR, Ayuningtyas NF, Wati SM, Rahayu RP, Urbina F, Kuntari WL, Syahnia SJMR, Puspasari K, et al. Pigmented Fungiform Papillae (PFP) of the Tongue: A Systematic Review of Current Aetiopathogenesis and Pathophysiology. Pathophysiology. 2022; 29(3):555-569. https://doi.org/10.3390/pathophysiology29030043
Chicago/Turabian StyleSurboyo, Meircurius Dwi Condro, Lakshman Samaranayake, Arvind Babu Rajendra Santosh, Nurina Febriyanti Ayuningtyas, Sisca Meida Wati, Retno Pudji Rahayu, Francisco Urbina, Winni Langgeng Kuntari, Sesaria Junita Mega Rahma Syahnia, Karlina Puspasari, and et al. 2022. "Pigmented Fungiform Papillae (PFP) of the Tongue: A Systematic Review of Current Aetiopathogenesis and Pathophysiology" Pathophysiology 29, no. 3: 555-569. https://doi.org/10.3390/pathophysiology29030043
APA StyleSurboyo, M. D. C., Samaranayake, L., Santosh, A. B. R., Ayuningtyas, N. F., Wati, S. M., Rahayu, R. P., Urbina, F., Kuntari, W. L., Syahnia, S. J. M. R., Puspasari, K., Parmadiati, A. E., & Ernawati, D. S. (2022). Pigmented Fungiform Papillae (PFP) of the Tongue: A Systematic Review of Current Aetiopathogenesis and Pathophysiology. Pathophysiology, 29(3), 555-569. https://doi.org/10.3390/pathophysiology29030043