Role of Candida albicans in Oral Carcinogenesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources and Search Strategy
2.2. Study Selection
2.3. Data Extraction
2.4. Data Synthesis and Analysis
3. Results
3.1. Study Characteristic
3.2. Candida Isolation and Culture Procedure
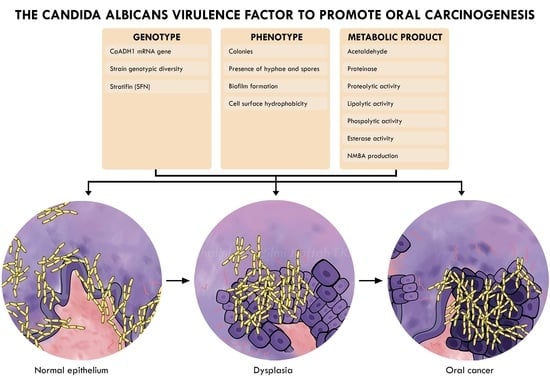
3.3. Candida Virulence Factor
3.3.1. Phenotype
3.3.2. Genotype
3.3.3. Metabolic Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Abati, S.; Bramati, C.; Bondi, S.; Lissoni, A.; Trimarchi, M. Oral Cancer and Precancer: A Narrative Review on the Relevance of Early Diagnosis. Int. J. Environ. Res. Public Health 2020, 17, 9160. [Google Scholar] [CrossRef]
- Markopoulos, A.K. Current Aspects on Oral Squamous Cell Carcinoma. Open Dent. J. 2012, 6, 126–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vargas-Ferreira, F.; Nedel, F.; Etges, A.; Gomes, A.P.N.; Furuse, C.; Tarquinio, S.B.C. Etiologic factors associated with oral squamous cell carcinoma in non-smokers and non-alcoholic drinkers: A brief approach. Braz. Dent. J. 2012, 23, 586–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shrestha, A.D.; Vedsted, P.; Kallestrup, P.; Neupane, D. Prevalence and incidence of oral cancer in low- and middle-income countries: A scoping review. Eur. J. Cancer Care 2019, 29, e13207. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Arakeri, G.; Alamir, A.W.H.; Patil, S.; Awan, K.H.; Baeshen, H.; Raj, T.; Fonseca, F.P.; Brennan, P.A. Is toombak a risk factor for oral leukoplakia and oral squamous cell carcinoma? A systematic review. J. Oral Pathol. Med. 2020, 49, 103–109. [Google Scholar] [CrossRef]
- Falzone, L.; Marconi, A.; Loreto, C.; Franco, S.; Spandidos, D.A.; Libra, M. Occupational exposure to carcinogens: Benzene, pesticides and fibers. Mol. Med. Rep. 2016, 14, 4467–4474. [Google Scholar] [CrossRef] [Green Version]
- Malfa, G.A.; Tomasello, B.; Sinatra, F.; Villaggio, G.; Amenta, F.; Avola, R.; Renis, M. “Reactive” response evaluation of primary human astrocytes after methylmercury exposure. J. Neurosci. Res. 2014, 92, 95–103. [Google Scholar] [CrossRef]
- Rapisarda, V.; Ledda, C.; Matera, S.; Fago, L.; Arrabito, G.; Falzone, L.; Marconi, A.; Libra, M.; Loreto, C. Absence of t(14;18) chromosome translocation in agricultural workers after short-term exposure to pesticides. Mol. Med. Rep. 2017, 15, 3379–3382. [Google Scholar] [CrossRef] [Green Version]
- Karpiński, T.M. Role of Oral Microbiota in Cancer Development. Microorganisms 2019, 7, 20. [Google Scholar] [CrossRef] [Green Version]
- Pang, X.; Tang, Y.J.; Ren, X.H.; Chen, Q.M.; Tang, Y.L.; Liang, X.H. Microbiota, Epithelium, Inflammation, and TGF-β Signaling: An Intricate Interaction in Oncogenesis. Front. Microbiol. 2018, 9, 1353. [Google Scholar] [CrossRef]
- Multhoff, G.; Molls, M.; Radons, J. Chronic Inflammation in Cancer Development. Front. Immunol. 2012, 2, 98. [Google Scholar] [CrossRef] [Green Version]
- Alnuaimi, A.; Wiesenfeld, D.; O’Brien-Simpson, N.; Reynolds, E.; Peng, B.; McCullough, M. The development and validation of a rapid genetic method for species identification and genotyping of medically important fungal pathogens using high-resolution melting curve analysis. Mol. Oral Microbiol. 2014, 29, 117–130. [Google Scholar] [CrossRef]
- Yete, S.; D’Souza, W.; Saranath, D. High-Risk Human Papillomavirus in Oral Cancer: Clinical Implications. Oncology 2018, 94, 133–141. [Google Scholar] [CrossRef]
- Muzio, L.L.; Ballini, A.; Cantore, S.; Bottalico, L.; Charitos, I.A.; Ambrosino, M.; Nocini, R.; Malcangi, A.; Dioguardi, M.; Cazzolla, A.P.; et al. Overview of Candida albicans and Human Papillomavirus (HPV) Infection Agents and their Biomolecular Mechanisms in Promoting Oral Cancer in Pediatric Patients. Biomed. Res. Int. 2021, 2021, 7312611. [Google Scholar] [CrossRef]
- Mallika, L.; Augustine, D.; Rao, R.S.; Patil, S.; Alamir, A.W.H.; Awan, K.H.; Sowmya, S.V.; Haragannavar, V.C.; Prasad, K. Does microbiome shift play a role in carcinogenesis? A systematic review. Transl. Cancer Res. 2020, 9, 3153–3166. [Google Scholar] [CrossRef]
- Williams, D.W.; Bartie, K.L.; Potts, A.; Wilson, M.J.; Fardy, M.J.; Lewis, M. Strain persistence of invasive Candida albican in chronic hyperplastic candidosis that underwent malignant change. Gerodontology 2001, 18, 73–78. [Google Scholar] [CrossRef]
- Cannon, R.D.; Chaffin, W.L. Oral Colonization By Candida albicans. Crit. Rev. Oral Biol. Med. 1999, 10, 359–383. [Google Scholar] [CrossRef]
- Rodríguez, M.J.; Schneider, J.; Moragues, M.-D.; Martínez-Conde, R.; Pontón, J.; Aguirre, J.M. Cross-reactivity between Candida albicans and oral squamous cell carcinoma revealed by monoclonal antibody C7. Anticancer Res. 2007, 27, 3639–3643. [Google Scholar]
- McManus, B.A.; Coleman, D.C. Molecular epidemiology, phylogeny and evolution of Candida albicans. Infect. Genet. Evol. 2014, 21, 166–178. [Google Scholar] [CrossRef] [Green Version]
- van der Waal, I. Potentially malignant disorders of the oral and oropharyngeal mucosa; terminology, classification and present concepts of management. Oral Oncol. 2009, 45, 317–323. [Google Scholar] [CrossRef]
- Arya, C.P.; Jaiswal, R.; Tandon, A.; Jain, A. Isolation and identification of oral Candida species in potentially malignant disorder and oral squamous cell carcinoma. Natl. J. Maxillofac. Surg. 2021, 12, 387. [Google Scholar] [CrossRef] [PubMed]
- Tamgadge, S.; Tamgadge, A.; Pillai, A.; Chande, M.; Acharya, S.; Kamat, N. Association of Candida sp. with the Degrees of Dysplasia and Oral Cancer: A Study by Calcofluor White under Fluorescent Microscopy. Iran J. Pathol. 2017, 12, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Hafed, L.; Farag, H.; El-Rouby, D.; Shaker, O.; Shabaan, H.-A. Candida Albicans Alcohol Dehydrogenase 1 gene in oral dysplasia and oral squamous cell carcinoma. Pol. J. Pathol. 2019, 70, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Alnuaimi, A.D.; Wiesenfeld, D.; O’Brien-Simpson, N.; Reynolds, E.; McCullough, M.J. Oral Candida colonization in oral cancer patients and its relationship with traditional risk factors of oral cancer: A matched case-control study. Oral Oncol. 2015, 51, 139–145. [Google Scholar] [CrossRef]
- Krogh, P.; Hald, B.; Holmstrup, P. Possible mycological etiology of oral mucosal cancer: Catalytic potential of infecting Candida aibicans and other yeasts in production of N-nitrosobenzylmethylamine. Carcinogenesis 1987, 8, 1543–1548. [Google Scholar] [CrossRef] [PubMed]
- Del Valle Castillo, G.; de Blanc, S.L.; Sotomayor, C.E.; Azcurra, A.I. Study of virulence factor of Candida species in oral lesions and its association with potentially malignant and malignant lesions. Arch. Oral Biol. 2018, 91, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Gainza-Cirauqui, M.L.; Nieminen, M.T.; Novak Frazer, L.; Aguirre-Urizar, J.M.; Moragues, M.D.; Rautemaa, R. Production of carcinogenic acetaldehyde by Candida albicans from patients with potentially malignant oral mucosal disorders. J. Oral Pathol. Med. 2013, 42, 243–249. [Google Scholar] [CrossRef]
- Nawaz, A.; Mäkinen, A.; Pärnänen, P.; Meurman, J.H. Proteolytic activity of non-albicans Candida and Candida albicans in oral cancer patients. New Microbiol. 2018, 41, 296–301. [Google Scholar]
- Alnuaimi, A.D.; Ramdzan, A.N.; Wiesenfeld, D.; O’Brien-Simpson, N.M.; Kolev, S.D.; Reynolds, E.C.; McCullough, M.J. Candida virulence and ethanol-derived acetaldehyde production in oral cancer and non-cancer subjects. Oral Dis. 2016, 22, 805–814. [Google Scholar] [CrossRef]
- Sankari, S.L.; Mahalakshmi, K.; Kumar, V.N. A comparative study of Candida species diversity among patients with oral squamous cell carcinoma and oral potentially malignant disorders. BMC Res. Notes 2020, 13, 488. [Google Scholar] [CrossRef]
- Abidullah, M.; Bhosle, S.; Komire, B.; Sharma, P.; Swathi, K.; Karthik, L. Investigation of Candidal Species among People Who Suffer from Oral Potentially Malignant Disorders and Oral Squamous Cell Carcinoma. J. Pharm. Bioallied. Sci. 2021, 13 (Suppl. 2), S1050–S1054. [Google Scholar]
- Hsieh, Y.-P.; Wu, Y.-H.; Cheng, S.-M.; Lin, F.-K.; Hwang, D.-Y.; Jiang, S.-S.; Chen, K.-C.; Chen, M.-Y.; Chiang, W.-F.; Liu, K.-J.; et al. Single-Cell RNA Sequencing Analysis for Oncogenic Mechanisms Underlying Oral Squamous Cell Carcinoma Carcinogenesis with Candida albicans Infection. Int. J. Mol. Sci. 2022, 23, 4833. [Google Scholar] [CrossRef]
- Sankari, S.L.; Mahalakshmi, K. Oral Candidal Carriage Among Patients with Oral Potential Malignant Disorders: A Case-Control Study. Pesqui. Bras. Odontopediatr. Clin. Integr. 2019, 19, e4802. [Google Scholar] [CrossRef]
- Gallè, F.; Colella, G.; Di Onofrio, V.; Rossiello, R.; Angelillo, I.F.; Liguori, G. Candida spp. in oral cancer and oral precancerous lesions. New Microbiol. 2013, 36, 283–288. [Google Scholar]
- Di Cosola, M.; Cazzolla, A.P.; Charitos, I.A.; Ballini, A.; Inchingolo, F.; Santacroce, L. Candida albicans and oral carcinogenesis. A brief review. J. Fungi. 2021, 7, 476. [Google Scholar] [CrossRef]
- Casadevall, A.; Pirofski, L.-A. What Is a Host? Incorporating the Microbiota into the Damage-Response Framework. Infect. Immun. 2015, 83, 2–7. [Google Scholar] [CrossRef] [Green Version]
- Pirofski, L.-A.; Casadevall, A. The Damage–Response Framework as a Tool for the Physician-Scientist to Understand the Pathogenesis of Infectious Diseases. J. Infect. Dis. 2018, 218 (Suppl. 1), S7–S11. [Google Scholar] [CrossRef] [Green Version]
- Pirofski, L.-A.; Casadevall, A. The Damage-Response Framework of Microbial Pathogenesis and Infectious Diseases. Adv. Exp. Med. Biol. 2008, 635, 135–146. [Google Scholar]
- Ruiz-Herrera, J.; Elorza, M.V.; Valentín, E.; Sentandreu, R. Molecular organization of the cell wall of Candida albicans and its relation to pathogenicity. FEMS Yeast Res. 2006, 6, 14–29. [Google Scholar] [CrossRef] [Green Version]
- Mishra, P.; Bolard, J.; Prasad, R. Emerging role of lipids of Candida albicans, a pathogenic dimorphic yeast. Biochim. Biophys. Acta 1992, 1127, 1–14. [Google Scholar] [CrossRef]
- Forche, A.; Alby, K.; Schaefer, D.; Johnson, A.D.; Berman, J.; Bennett, R.J. The Parasexual Cycle in Candida albicans Provides an Alternative Pathway to Meiosis for the Formation of Recombinant Strains. PLoS Biol. 2008, 6, e110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, N.; Upritchard, J.E.; Holland, B.; Fenton, L.E.; Ferguson, M.M.; Cannon, R.; Schmid, J. Distribution of mutations distinguishing the most prevalent disease-causing Candida albicans genotype from other genotypes. Infect. Genet. Evol. 2009, 9, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, M.L.; Limon, J.J.; Underhill, D.M. Immunity to Commensal Fungi: Detente and Disease. Annu. Rev. Pathol. Mech. Dis. 2017, 12, 359–385. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jönsson, F. Expression, Role, and Regulation of Neutrophil Fcγ Receptors. Front. Immunol. 2019, 10, 1958. [Google Scholar] [CrossRef] [PubMed]
- Černáková, L.; Rodrigues, C.F. Microbial interactions and immunity response in oral Candida species. Future Microbiol. 2020, 15, 1653–1677. [Google Scholar] [CrossRef]
- McCullough, M.; Jaber, M.; Barrett, A.; Bain, L.; Speight, P.; Porter, S. Oral yeast carriage correlates with presence of oral epithelial dysplasia. Oral Oncol. 2002, 38, 391–393. [Google Scholar] [CrossRef]
- Nagy, K. The microflora associated with human oral carcinomas. Oral Oncol. 1998, 34, 304–308. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of Invasive Candidiasis: A Persistent Public Health Problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef] [Green Version]
- Tobudic, S.; Kratzer, C.; Lassnigg, A.; Graninger, W.; Presterl, E. In vitro activity of antifungal combinations against Candida albicans biofilms. J. Antimicrob. Chemother. 2010, 65, 271–274. [Google Scholar] [CrossRef] [Green Version]
- McCullough, M.J.; Clemons, K.V.; Stevens, D.A. Molecular and Phenotypic Characterization of Genotypic Candida albicans Subgroups and Comparison with Candida dubliniensis and Candida stellatoidea. J. Clin. Microbiol. 1999, 37, 417–421. [Google Scholar] [CrossRef] [Green Version]
- Gayathri, K.; Balachander, N.; Malathi, L.; Sankari, S. Candida in potentially malignant oral disorders. J. Pharm. Bioallied Sci. 2015, 7, 162–164. [Google Scholar] [CrossRef]
- Khongsti, S.; Shunyu, B.; Ghosh, S. Promoter-associated DNA methylation & expression profiling of genes (FLT 3, EPB41L3 & SFN) in patients with oral squamous cell carcinoma in the Khasi & Jaintia population of Meghalaya, India. Indian J. Med. Res. 2019, 150, 584. [Google Scholar]
- Hu, Y.; Zeng, Q.; Li, C.; Xie, Y. Expression profile and prognostic value of SFN in human ovarian cancer. Biosci. Rep. 2019, 39, BSR20190100. [Google Scholar] [CrossRef] [Green Version]
- Rishehri, M.; Etemadi, T.; Pisheh, L.; Koufigar, G.; Azadeh, M. Quantitative Expression of SFN, lncRNA CCDC18-AS1, and lncRNA LINC01343 in Human Breast Cancer as the Regulator Biomarkers in a Novel ceRNA Network: Based on Bioinformatics and Experimental Analyses. Genet. Res. 2022, 2022, 6787791. [Google Scholar] [CrossRef]
- Chauhan, S.; Sen, S.; Chauhan, S.S.; Pushker, N.; Tandon, R.; Kashyap, S.; Vanathi, M.; Bajaj, M.S. Stratifin in ocular surface squamous neoplasia and its association with p53. Acta Ophthalmol. 2021, 99, e1483–e1491. [Google Scholar] [CrossRef]
- Ma, Y.-S.; Hou, L.-K.; Yao, S.-H.; Liu, J.-B.; Yu, X.-C.; Shi, Y.; Yang, X.-L.; Wu, W.; Wu, C.-Y.; Jiang, G.-X.; et al. Elevated Stratifin promotes cisplatin-based chemotherapy failure and poor prognosis in non-small cell lung cancer. Mol. Ther. Oncolytics 2021, 22, 326–335. [Google Scholar] [CrossRef]
- Homann, N. High acetaldehyde levels in saliva after ethanol consumption: Methodological aspects and pathogenetic implications. Carcinogenesis 1997, 18, 1739–1743. [Google Scholar] [CrossRef]
- Homann, N.; Tillonen, J.; Meurman, J.; Rintamäki, H.; Lindqvist, C.; Rautio, M.; Jousimies-Somer, H.; Salaspuro, M. Increased salivary acetaldehyde levels in heavy drinkers and smokers: A microbiological approach to oral cavity cancer. Carcinogenesis 2000, 21, 663–668. [Google Scholar] [CrossRef]
- Uittamo, J.; Siikala, E.; Kaihovaara, P.; Salaspuro, M.; Rautemaa, R. Chronic candidosis and oral cancer in APECED-patients: Production of carcinogenic acetaldehyde from glucose and ethanol by Candida albicans. Int. J. Cancer 2009, 124, 754–756. [Google Scholar] [CrossRef]
- Cheng, R.; Li, D.; Shi, X.; Gao, Q.; Wei, C.; Li, X.; Li, Y.; Zhou, H. Reduced CX3CL1 Secretion Contributes to the Susceptibility of Oral Leukoplakia-Associated Fibroblasts to Candida albicans. Front. Cell. Infect. Microbiol. 2016, 6, 150. [Google Scholar] [CrossRef] [Green Version]
- Kabir, M.A.; Hussain, M.A.; Ahmad, Z. Candida albicans: A Model Organism for Studying Fungal Pathogens. ISRN Microbiol. 2012, 2012, 538694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramirez-Garcia, A.; Rementeria, A.; Aguirre-Urizar, J.M.; Moragues, M.D.; Antoran, A.; Pellon, A.; Abad-Diaz-de-Cerio, A.; Hernando, F.L. Candida albicans and cancer: Can this yeast induce cancer development or progression? Crit. Rev. Microbiol. 2014, 42, 93–181. [Google Scholar] [PubMed]
- Sardi, J.C.O.; Duque, C.; Höfling, J.F.; Gonçalves, R.B. Genetic and phenotypic evaluation of Candida albicans strains isolated from subgingival biofilm of diabetic patients with chronic periodontitis. Med. Mycol. 2012, 50, 467–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurago, Z.B.; Lam-Ubol, A.; Stetsenko, A.; De La Mater, C.; Chen, Y.; Dawson, D.V. Lipopolysaccharide-Squamous Cell Carcinoma-Monocyte Interactions Induce Cancer-Supporting Factors Leading to Rapid STAT3 Activation. Head Neck Pathol. 2008, 2, 1–12. [Google Scholar] [CrossRef]
- Lax, A.J. Bacterial toxins and cancer—A case to answer? Nat. Rev. Microbiol. 2005, 3, 343–349. [Google Scholar] [CrossRef]
- Sanjaya, P.; Gokul, S.; Patil, B.G.; Raju, R. Candida in oral pre-cancer and oral cancer. Med. Hypotheses 2011, 77, 1125–1128. [Google Scholar] [CrossRef]

| Country | Study Design | Case Groups (n) | Control Groups (n) | Reference |
|---|---|---|---|---|
| India | Retrospective analytic cross sectional |
| Healthy gingiva (20) | [22] |
| Egypt | Retrospective analytic cross sectional |
| Healthy gingiva (7) | [23] |
| Australia | Case-control | Oral cancer (52) | Healthy mucosa (104) | [24] |
| Denmark | Analytic cross-sectional | Leukoplakia and erythroplakia (12) | NA | [25] |
| Argentina | Analytic cross-sectional |
| Asymptomatic Candida spp. carriers with healthy mucosa (15) | [26] |
| Spain | Analytic cross-sectional |
| Healthy mucosa with positive Candida albicans (6) | [27] |
| Finland | Analytic cross-sectional |
| NA | [28] |
| Australia | Analytic cross-sectional | Oral cancer (52) | Non-oral cancer patients (104) | [29] |
| India | Analytic cross-sectional |
| Healthy mucosa (200) | [30] |
| India | Prospective and observational study |
| Healthy mucosa (50) | [31] |
| Taiwan | Analytic cross-sectional |
| Health gingiva b | [32] |
| Sampling Methods | Detection Methos | Reference |
|---|---|---|
| Tissue biopsy | HE staining then examined under fluorescent microscopy | [22] |
| Tissue biopsy | HE staining then examined under fluorescent microscopy | [23] |
| Oral rinse | Culture in CHROM-agar Candida medium | [24] |
| Tissue biopsy and swab |
| [25] |
| Swab |
| [26] |
| Swab |
| [27] |
| Saliva collecting |
| [28] |
| Oral rinse |
| [29] |
| Saliva collecting |
| [30] |
| Swab |
| [31] |
| Tissue biopsy and swab |
| [32] |
| Phenotype Marker | Result | Reference |
|---|---|---|
| The presences of hyphae and spore |
| [22] |
| Colonies |
| [24] |
| [30] | |
| [31] | |
| Biofilm formation |
| [26] |
| [29] | |
| CSH |
| [26] |
| Genotype Marker | Result | Reference |
|---|---|---|
| CaADH1 mRNA gene |
| [23] |
| Strain genotypic | The oral cancer tissue is dominated by Candida albicans genotype A | [24] |
| SFN |
| [32] |
| Genotype Marker | Result | Reference |
|---|---|---|
| Acetaldehyde |
| [27] |
| [29] | |
| Proteinase |
| [29] |
| Proteolytic activity |
| [26] |
| The oral cancer with Candida albicans showed lower proteolytic activity than Candida tropicalis as non-albicans strain. | [28] | |
| Lipolytic activity |
| [26] |
| Phospolytic activity |
| [29] |
| Esterase activity |
| [29] |
| NMBA production | The erythroplakia and leukoplakia with Candida albicans exhibited the highest nitrosation production. | [25] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayuningtyas, N.F.; Mahdani, F.Y.; Pasaribu, T.A.S.; Chalim, M.; Ayna, V.K.P.; Santosh, A.B.R.; Santacroce, L.; Surboyo, M.D.C. Role of Candida albicans in Oral Carcinogenesis. Pathophysiology 2022, 29, 650-662. https://doi.org/10.3390/pathophysiology29040051
Ayuningtyas NF, Mahdani FY, Pasaribu TAS, Chalim M, Ayna VKP, Santosh ABR, Santacroce L, Surboyo MDC. Role of Candida albicans in Oral Carcinogenesis. Pathophysiology. 2022; 29(4):650-662. https://doi.org/10.3390/pathophysiology29040051
Chicago/Turabian StyleAyuningtyas, Nurina Febriyanti, Fatma Yasmin Mahdani, Togu Andrie Simon Pasaribu, Muhammad Chalim, Visilmi Kaffah Putri Ayna, Arvind Babu Rajendra Santosh, Luigi Santacroce, and Meircurius Dwi Condro Surboyo. 2022. "Role of Candida albicans in Oral Carcinogenesis" Pathophysiology 29, no. 4: 650-662. https://doi.org/10.3390/pathophysiology29040051
APA StyleAyuningtyas, N. F., Mahdani, F. Y., Pasaribu, T. A. S., Chalim, M., Ayna, V. K. P., Santosh, A. B. R., Santacroce, L., & Surboyo, M. D. C. (2022). Role of Candida albicans in Oral Carcinogenesis. Pathophysiology, 29(4), 650-662. https://doi.org/10.3390/pathophysiology29040051