Bioethanol Production from Cachaza as Hydrogen Feedstock: Effect of Ammonium Sulfate during Fermentation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Ethanol and Aliphatic Production during Fermentation of Glucose
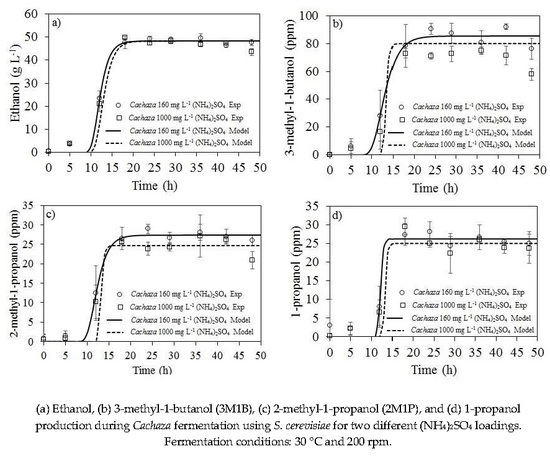
2.2. Ethanol and Aliphatic Production during Fermentation of Cachaza
2.3. Kinetic Parameters Estimation for Biomass, Ethanol, 1-Propanol, and Branched Alcohol Production during Fermentation of Cachaza
3. Materials and Methods
3.1. Cachaza Collection
3.2. Yeast Activation and Inoculum Preparation
3.3. Ammonium Sulfate Screening
3.4. Cachaza Fermentation
3.5. Analytical Methods
3.6. Kinetic Parameter Estimation
3.7. Experimental Design and Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kirubakaran, A.; Jain, S.; Nema, R.K. A review on fuel cell technologies and power electronic interface. Renew. Sustain. Energy Rev. 2009, 13, 2430–2440. [Google Scholar] [CrossRef]
- Mekhilef, S.; Saidur, R.; Safari, A. Comparative study of different fuel cell technologies. Renew. Sustain. Energy Rev. 2012, 16, 981–989. [Google Scholar] [CrossRef]
- Sanchez, N.; Ruiz, R.Y.; Cifuentes, B.; Cobo, M. Hydrogen from glucose: A combined study of glucose fermentation, bioethanol purification, and catalytic steam reforming. Int. J. Hydrogen Energy 2016, 41, 5640–5651. [Google Scholar] [CrossRef]
- Cifuentes, B.; Figueredo, M.; Cobo, M. Response Surface Methodology and Aspen Plus Integration for the Simulation of the Catalytic Steam Reforming of Ethanol. Catalysts 2017, 7, 15. [Google Scholar] [CrossRef]
- Ansari, K.B.; Gaikar, V.G. Pressmud as an Alternate Resource for Hydrocarbons and Chemicals by Thermal Pyrolysis. Ind. Eng. Chem. Res. 2014, 53, 1878–1889. [Google Scholar] [CrossRef]
- López-González, L.M.; Pereda Reyes, I.; Dewulf, J.; Budde, J.; Heiermann, M.; Vervaeren, H. Effect of liquid hot water pre-treatment on sugarcane press mud methane yield. Bioresour. Technol. 2014, 169, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Janke, L.; Leite, A.F.; Nikolausz, M.; Radetski, C.M.; Nelles, M.; Stinner, W. Comparison of start-up strategies and process performance during semi-continuous anaerobic digestion of sugarcane filter cake co-digested with bagasse. Waste Manag. 2016, 48, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Janke, L.; Leite, A.; Batista, K.; Weinrich, S.; Sträuber, H.; Nikolausz, M. Optimization of hydrolysis and volatile fatty acids production from sugarcane filter cake: Effects of urea supplementation and sodium hydroxide pretreatment. Bioresour. Technol. 2016, 199, 235–244. [Google Scholar] [CrossRef] [PubMed]
- George, P.A.O.; Eras, J.J.C.; Gutierrez, A.S.; Hens, L.; Vandecasteele, C. Residue from Sugarcane Juice Filtration (Filter Cake): Energy Use at the Sugar Factory. Waste Biomass Valoriz. 2010, 1, 407–413. [Google Scholar] [CrossRef]
- Kuruti, K.; Rao, A.G.; Gandu, B.; Kiran, G.; Mohammad, S.; Sailaja, S.; Swamy, Y.V. Generation of bioethanol and VFA through anaerobic acidogenic fermentation route with press mud obtained from sugar mill as a feedstock. Bioresour. Technol. 2015, 192, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Radjaram, B.; Saravanane, R. Start up study of UASB reactor treating press mud for biohydrogen production. Biomass Bioenergy 2011, 35, 2721–2728. [Google Scholar] [CrossRef]
- Puligundla, P.; Smogrovicova, D.; Obulam, V.S.R.; Ko, S. Very high gravity (VHG) ethanolic brewing and fermentation: A research update. J. Ind. Microbiol. Biotechnol. 2011, 38, 1133–1144. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, Y.; Xu, G.; Chu, J.; Zhuang, Y.; Zhang, S. Influence of high solid concentration on enzymatic hydrolysis and fermentation of steam-exploded corn stover biomass. Appl. Biochem. Biotechnol. 2010, 160, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, P.F.; Karp, S.G.; Carvalho, J.C.; Sturm, W.; Rodríguez-León, J.A.; Tholozan, J.L.; Singhania, R.R.; Pandey, A.; Soccol, C.R. Production of bio-ethanol from soybean molasses by Saccharomyces cerevisiae at laboratory, pilot and industrial scales. Bioresour. Technol. 2008, 99, 8156–8163. [Google Scholar] [CrossRef] [PubMed]
- Alvira, P.; Moreno, A.D.; Ibarra, D.; Sáez, F.; Ballesteros, M. Improving the fermentation performance of Saccharomyces cerevisiae by laccase during ethanol production from steam-exploded wheat straw at high-substrate loadings. Biotechnol. Prog. 2013, 29, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, D.; Shi, Y. Effects of nitrogen source on ethanol production in very high gravity fermentation of corn starch. J. Taiwan Inst. Chem. Eng. 2017, 70, 229–235. [Google Scholar] [CrossRef]
- Betite, V.C.; Júnior, M.M.; de Oliveira, J.E.; Ernandes, J.R. Very high gravity sucrose fermentation by brazilian industrial yeast strains: Effect of nitroge supplementation. J. Inst. Brew. 2012, 118, 174–178. [Google Scholar] [CrossRef]
- Hu, C.; Qin, Q.; Gao, P. Medium optimization for improved ethanol production in very high gravity fermentation. Chin. J. Chem. Eng. 2011, 19, 1017–1022. [Google Scholar] [CrossRef]
- Le Valant, A.; Garron, A.; Bion, N.; Epron, F.; Duprez, D. Hydrogen production from raw bioethanol over Rh/MgAl2O4 catalyst. Catal. Today 2008, 138, 169–174. [Google Scholar] [CrossRef]
- Le Valant, A.; Can, F.; Bion, N.; Duprez, D.; Epron, F. Hydrogen production from raw bioethanol steam reforming: Optimization of catalyst composition with improved stability against various impurities. Int. J. Hydrogen Energy 2010, 35, 5015–5020. [Google Scholar] [CrossRef]
- Rass-Hansen, J.; Johansson, R.; Møller, M.; Christensen, C.H. Steam reforming of technical bioethanol for hydrogen production. Int. J. Hydrogen Energy 2008, 33, 4547–4554. [Google Scholar] [CrossRef]
- Devianto, H.; Han, J.; Yoon, S.P.; Nam, S.W.; Lim, T.H.; Oh, I.H.; Hong, S.A.; Lee, H.I. The effect of impurities on the performance of bioethanol-used internal reforming molten carbonate fuel cell. Int. J. Hydrogen Energy 2011, 36, 10346–10354. [Google Scholar] [CrossRef]
- Dan, M.; Senila, L.; Roman, M.; Mihet, M.; Lazar, M.D. From wood wastes to hydrogen—Preparation and catalytic steam reforming of crude bio-ethanol obtained from fir wood. Renew. Energy 2015, 74, 27–36. [Google Scholar] [CrossRef]
- Vargas, J.C.; Libs, S.; Roger, A.-C.; Kiennemann, A. Study of Ce-Zr-Co fluorite-type oxide as catalysts for hydrogen production by steam reforming of bioethanol. Catal. Today 2005, 107–108, 417–425. [Google Scholar] [CrossRef]
- Deed, N.K.; Van Vuuren, H.J.J.; Gardner, R.C. Effects of nitrogen catabolite repression and di-ammonium phosphate addition during wine fermentation by a commercial strain of S. cerevisiae. Appl. Microbiol. Biotechnol. 2011, 89, 1537–1549. [Google Scholar] [CrossRef] [PubMed]
- Aranda, A.; Matallana, E.; Del Olmo, M. Saccharomyces Yeasts I: Primary Fermentation; Elsevier: Oxford, UK, 2011. [Google Scholar]
- Comelli, N.; Seluy, L.G.; Isla, M.A. Optimization of a low-cost defined medium for alcoholic fermentation—A case study for potential application in bioethanol production from industrial wastewaters. New Biotechnol. 2016, 33, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Vidal, E.E.; De Billerbeck, G.M.; Simões, D.A.; Schuler, A.; François, J.M.; De Morais, M.A. Influence of nitrogen supply on the production of higher alcohols/esters and expression of flavour-related genes in cachaça fermentation. Food Chem. 2013, 138, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Orte, P.; Bely, M.; Cacho, J.; Ferreira, V. Impact of ammonium additions on volatile acidity, ethanol, and aromatic compound production by different Saccharomyces cerevisiae strains during fermentation in controlled synthetic media. Aust. J. Grape Wine Res. 2006, 12, 150–160. [Google Scholar] [CrossRef]
- Beltran, G.; Esteve-Zarzoso, B.; Rozès, N.; Mas, A.; Guillamón, J.M. Influence of the timing of nitrogen additions during synthetic grape must fermentations on fermentation kinetics and nitrogen consumption. J. Agric. Food Chem. 2005, 53, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Nordström, K. Formation of esters, acids and alcohol from a-keto acids by brewer’s yeast. J. Inst. Brew. 1963, 69, 483–495. [Google Scholar] [CrossRef]
- Clement, T.; Perez, M.; Mouret, J.R.; Sanchez, I.; Sablayrolles, J.M.; Camarasa, C. Metabolic responses of Saccharomyces cerevisiae to valine and ammonium pulses during four-stage continuous wine fermentations. Appl. Environ. Microbiol. 2013, 79, 2749–2758. [Google Scholar] [CrossRef] [PubMed]
- Phukoetphim, N.; Salakkam, A.; Laopaiboon, P. Kinetic models for batch ethanol production from sweet sorghum juice under normal and high gravity fermentations: Logistic and modified Gompertz models. J. Biotechnol. 2017, 243, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.; Wang, S.; Xing, L.; Li, B.; Peng, Y. The effect of salinity on waste activated sludge alkaline fermentation and kinetic analysis. J. Environ. Sci. 2015, 3, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Hazelwood, L.A.; Daran, J.M.; van Maris, A.J.A.; Pronk, J.T.; Dickinson, J.R. The ehrlich pathway for fusel alcohol production: A century of research on Saccharomyces cerevisiae metabolism. Appl. Environ. Microbiol. 2008, 74, 2259–2266. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, A.; Chiva, R.; Sancho, M.; Beltran, G.; Arroyo-López, F.N.; Guillamon, J.M. Nitrogen requirements of commercial wine yeast strains during fermentation of a synthetic grape must. Food Microbiol. 2012, 31, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Crépin, L.; Nidelet, T.; Sanchez, I.; Dequin, S.; Camarasa, C. Sequential Use of Nitrogen Compounds by Saccharomyces cerevisiae during Wine Fermentation: A Model Based on Kinetic and Regulation Characteristics of Nitrogen Permeases. Appl. Environ. Microbiol. 2012, 78, 8102–8111. [Google Scholar] [CrossRef] [PubMed]
- Pires, E.J.; Teixeira, J.A.; Brányik, T.; Vicente, A.A. Yeast: The soul of beer’s aroma—A review of flavour-active esters and higher alcohols produced by the brewing yeast. Appl. Microbiol. Biotechnol. 2014, 98, 1937–1949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atsumi, S.; Hanai, T.; Liao, J.C. Non-fermentative pathways for synthesis of branched-chain higher alcohols as biofuels. Nature 2008, 451, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Pietruszka, M.; Katarzyna, P.-P.; Józef, S.S. Synthesis of higher alcohols during alcoholic fermentation of rye mashes. Food Chem. Biotechnol. 2010, 74, 51–63. [Google Scholar]
- Reazin, G.; Scales, H.; Andreasen, A. Production of higher alcohols from threonine and isoleucine in alcoholic fermentations of different types of grain mash. J. Agric. Food Chem. 1973, 21, 50–54. [Google Scholar] [CrossRef]
- Renger, R.S.; Van Hateren, S.H.; Luyben, K.C.A.M. The formation of esters and higher alcohols during brewery fermentation: The effect of carbon dioxide pressure. J. Inst. Brew. 1992, 98, 509–513. [Google Scholar] [CrossRef]
- Sanchez, N.; Gomez, M.; Ruíz, R.; Plazas, A.; Vásquez, J.; Cobo, M. Effect of the hydrolysis Pretreatment of Cachaza for Bioethanol Production. In Proceedings of the 25th European Biomass Conference and Exhibition, Stockholm, Sweden, 12–15 June 2017. [Google Scholar]
- Tanksale, A.; Beltramini, J.N.; Lu, G.M. A review of catalytic hydrogen production processes from biomass. Renew. Sustain. Energy Rev. 2010, 14, 166–182. [Google Scholar] [CrossRef]
- Modenbach, A.A.; Nokes, S.E. Enzymatic hydrolysis of biomass at high-solids loadings—A review. Biomass Bioenergy 2013, 56, 526–544. [Google Scholar] [CrossRef]
- Godard, P.; Urrestarazu, A.; Vissers, S.; Kontos, K.; Bontempi, G.; van Helden, J.; Andre, B. Effect of 21 Different Nitrogen Sources on Global Gene Expression in the Yeast Saccharomyces cerevisiae. Mol. Cell. Biol. 2007, 27, 3065–3086. [Google Scholar] [CrossRef] [PubMed]
- Dodic, J.M.; Vucurovic, D.G.; Dodic, S.N.; Grahovac, J.A.; Popov, S.D.; Nedeljkovic, N.M. Kinetic modelling of batch ethanol production from sugar beet raw juice. Appl. Energy 2012, 99, 192–197. [Google Scholar] [CrossRef]
- Baranyi, J.; Roberts, T.A. A dynamic approach to predicting bacterial growth in food. Int. J. Food Microbiol. 1994, 23, 277–294. [Google Scholar] [CrossRef]
- Membré, J.M.; Kubaczka, M.; Chéné, C. Combined effects of pH and sugar on growth rate of Zygosaccharomyces rouxii, a bakery product spoilage yeast. Appl. Environ. Microbiol. 1999, 65, 4921–4925. [Google Scholar] [PubMed]
- Boons, K.; Noriega, E.; Verherstraeten, N.; David, C.C.; Hofkens, J.; Van Impe, J.F. The effect of medium structure complexity on the growth of Saccharomyces cerevisiae in gelatin–dextran systems. Int. J. Food Microbiol. 2015, 199, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Ariyajaroenwong, P.; Laopaiboon, P.; Salakkam, A. Kinetic models for batch and continuous ethanol fermentation from sweet sorghum juice by yeast immobilized on sweet sorghum stalks. J. Taiwan Inst. Chem. Eng. 2016, 66, 210–216. [Google Scholar] [CrossRef]
- Lambrechts, M.G.; Pretorius, I.S. Yeast and its Importance to Wine Aroma—A Review. S. Afr. J. Enol. Vitic. 2000, 21, 97–129. [Google Scholar]
- Landaud, S.; Latrille, E.; Corrieu, G. Top pressure and temperature control the fusel alcohol/ester ratio through yeast growth in beer fermentation. J. Inst. Brew. 2001, 107, 107–117. [Google Scholar] [CrossRef]
- Zabed, H.; Faruq, G.; Sahu, J.N.; Azirun, M.S.; Hashim, R.; Nasrulhaq Boyce, A. Bioethanol production from fermentable sugar juice. Sci. World J. 2014, 2014, 957102. [Google Scholar] [CrossRef] [PubMed]
- Marks, V.D.; Van Der Merwe, G.K.; Van Vuuren, H.J.J. Transcriptional profiling of wine yeast in fermenting grape juice: Regulatory effect of diammonium phosphate. FEMS Yeast Res. 2003, 3, 269–287. [Google Scholar] [CrossRef]
- De Jong, E.; Higson, A.; Walsh, P.; Wellisch, M. Bio-Based Chemicals, Value Added Products from Biorefineries; IEA Bioenergy: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Goncalves, C.; Rodriguez-Jasso, R.M.; Gomes, N.; Teixeira, J.A.; Belo, I. Adaptation of dinitrosalicylic acid method to microtiter plates. Anal. Methods 2010, 2, 2046–2048. [Google Scholar] [CrossRef] [Green Version]
- Ross, T. Indices for performance evaluation of predictive models in food microbiology. J. Appl. Bacteriol. 1996, 81, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Baranyi, J.; Pin, C.; Ross, T. Validating and comparing predictive models. Int. J. Food Microbiol. 1999, 48, 159–166. [Google Scholar] [CrossRef]



| Parameters | N | E | EA | 1P | 2M1P | 1B | 3M1B | DW | CFU | XG |
|---|---|---|---|---|---|---|---|---|---|---|
| N | 0 | 0 | 0 | - | 0 | - | 0 | 0 | 0 | |
| E | 0 | + | + | 0 | 0 | 0 | 0 | 0 | + | |
| EA | 0 | + | + | 0 | 0 | 0 | 0 | 0 | + | |
| 1P | 0 | + | + | 0 | 0 | 0 | 0 | 0 | + | |
| 2M1B | - | 0 | 0 | 0 | 0 | + | 0 | 0 | 0 | |
| 1B | 0 | 0 | 0 | 0 | 0 | + | 0 | 0 | 0 | |
| 3M1B | - | 0 | 0 | 0 | + | + | 0 | 0 | 0 | |
| DW | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| CFU | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| XG | 0 | + | + | + | 0 | 0 | 0 | 0 | 0 |
| Parameter | Analytical Method | Method | wt % (Wet Basis) |
|---|---|---|---|
| Moisture | Gravimetric | AOAC * 925.45 Method B | 51.66 |
| Solid content | Calculus | Calculus | 48.34 |
| Total carbohydrates (including reducing sugar) | Calculus | Calculus | 41.10 |
| Reducing sugar | Volumetric | AOAC 923.09 | 28.50 |
| Wax | Gravimetric | AOAC 989.05 | 3.05 |
| Protein | Volumetric | AOAC 2001.11 | 2.35 |
| Ash | Gravimetric | AOAC 900.02 Method A | 1.84 |
| Nitrogen | Volumetric | AOAC 2001.11 | 0.38 |
| (NH4)2SO4 (mg·L−1) | Ethanol (g·L−1) | 2M1P (ppm) | 3M1B (ppm) | 1-Propanol (ppm) |
|---|---|---|---|---|
| 0 | 50.3 ± 1.8 (a) | 49.0 ± 4.0 (a) | 96.9 ± 7.5 (a) | 13.8 ± 0.4 (a) |
| 160 | 49.1 ± 1.1 (a) | 29.0 ± 1.1 (b) | 90.6 ± 3.9 (a) | 28.2 ± 2.5 (b) |
| 1000 | 47.3 ± 0.6 (a) | 23.8 ± 1.7 (c) | 71.1 ± 2.5 (b) | 25.1 ± 1.1 (b) |
| (NH4)2SO4 (mg·L−1) | μmax (h−1) | Lag Phase Time (h) | Final Value (g·L−1) |
|---|---|---|---|
| Glucose | |||
| 160 | 0.60 ± 0.08 (aA) | 6.01 ± 1.81 (aA) | 11.80 ± 0.23 (aA) |
| 1000 | 0.49 ± 0.01 (aA) | 4.92 ± 1.03 (aA) | 10.62 ± 0.68 (aA) |
| Cachaza | |||
| 160 | 0.75 ± 0.23 (aA) | 5.04 ± 1.69 (aA) | 10.00 ± 0.31 (aB) |
| 1000 | 0.75 ± 0.08 (aA) | 5.01 ± 0.83 (aA) | 10.13 ± 0.17 (aA) |
| (NH4)2SO4 Loading (mg·L−1) | Product | Pmax (g·L−1 (*) or ppm) | rp.m (g·L−1·h−1 (**) or ppm·h−1) | TL (h) | R2 | AF | BF | %Df | %Bf |
|---|---|---|---|---|---|---|---|---|---|
| 160 | Ethanol | 49.10 * | 3.93 ** | 3.36 | 0.969 | 1.618 | 0.977 | 61.8 | 0.88 |
| 3M1B | 85.42 | 14.14 | 10.03 | 0.926 | 1.062 | 1.001 | 6.2 | −0.05 | |
| 2M1P | 27.31 | 5.72 | 9.82 | 0.939 | 1.033 | 1.000 | 3.34 | −0.02 | |
| 1P | 26.20 | 23.35 | 11.66 | 0.881 | 1.048 | 1.001 | 4.85 | −0.06 | |
| 1000 | Ethanol | 50.24 * | 3.53 ** | 4.27 | 0.936 | 1.257 | 1.011 | 25.7 | 0.41 |
| 3M1B | 80.11 | 65.79 | 11.75 | 0.946 | 1.162 | 1.127 | 16.2 | 4.96 | |
| 2M1P | 24.66 | 20.67 | 11.51 | 0.924 | 1.080 | 1.003 | 8.0 | 0.11 | |
| 1P | 25.10 | 25.96 | 11.80 | 0.909 | 1.114 | 0.977 | 11.4 | −0.86 |
| Parameter | Value |
|---|---|
| Liquid to solid ratio (w/w) | 1:1 |
| Temperature (°C) | 90 |
| Time (h) | 3 |
| Acid content | 13.5 g H2SO4 per kg Cachaza (4.5 wt %) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez, N.; Ruiz, R.Y.; Infante, N.; Cobo, M. Bioethanol Production from Cachaza as Hydrogen Feedstock: Effect of Ammonium Sulfate during Fermentation. Energies 2017, 10, 2112. https://doi.org/10.3390/en10122112
Sanchez N, Ruiz RY, Infante N, Cobo M. Bioethanol Production from Cachaza as Hydrogen Feedstock: Effect of Ammonium Sulfate during Fermentation. Energies. 2017; 10(12):2112. https://doi.org/10.3390/en10122112
Chicago/Turabian StyleSanchez, Nestor, Ruth Yolanda Ruiz, Nicolas Infante, and Martha Cobo. 2017. "Bioethanol Production from Cachaza as Hydrogen Feedstock: Effect of Ammonium Sulfate during Fermentation" Energies 10, no. 12: 2112. https://doi.org/10.3390/en10122112
APA StyleSanchez, N., Ruiz, R. Y., Infante, N., & Cobo, M. (2017). Bioethanol Production from Cachaza as Hydrogen Feedstock: Effect of Ammonium Sulfate during Fermentation. Energies, 10(12), 2112. https://doi.org/10.3390/en10122112