The Role of Natural Laccase Redox Mediators in Simultaneous Dye Decolorization and Power Production in Microbial Fuel Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Operation of the Microbial Fuel Cell
2.2.1. Anode Chamber Composition
2.2.2. Cathode Chamber Composition
2.3. Experimental Design
2.4. Analytical Procedures
2.4.1. Acid Orange 7 Decolorization
2.4.2. Electrochemical Analysis
2.4.3. Cyclic Voltammetry (CV) of Redox Mediators
2.4.4. Chronoamperometry (CA) of Laccase-Mediators
2.4.5. Statistical Analysis
3. Results and Discussion
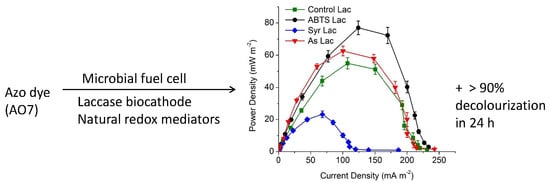
3.1. Power Generation
3.2. Acid Orange 7 Decolorization
3.3. Electrochemical Activity of the Laccase Mediator Systems
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Luo, H.; Jin, S.; Fallgren, P.H.; Park, H.J.; Johnson, P.A. A Novel Laccase-Catalyzed Cathode for Microbial Fuel Cells. Chem. Eng. J. 2010, 165, 524–528. [Google Scholar] [CrossRef]
- Savizi, I.S.P.; Kariminia, H.R.; Bakhshian, S. Simultaneous Decolorization and Bioelectricity Generation in a Dual Chamber Microbial Fuel Cell Using Electropolymerized-Enzymatic Cathode. Environ. Sci. Technol. 2012, 46, 6584–6593. [Google Scholar] [CrossRef] [PubMed]
- Galhaup, C.; Haltrich, D. Enhanced Formation of Laccase Activity by the White-Rot Fungus Trametes Pubescens in the Presence of Copper. Appl. Microbiol. Biotechnol. 2001, 56, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Alneyadi, A.H.; Rauf, M.A.; Ashraf, S.S. Oxidoreductases for the remediation of organic pollutants in water—A critical review. Crit. Rev. Biotechnol. 2018, 38, 971–988. [Google Scholar] [CrossRef] [PubMed]
- Morozova, O.V.; Shumakovich, G.P.; Shleev, S.V.; Yaropolov, Y.I. Laccase-Mediator systems and their Applications: A Review. Appl. Biochem. Microbiol. 2007, 43, 523–535. [Google Scholar] [CrossRef]
- Christopher, L.P.; Yao, B.; Ji, Y. Lignin Biodegradation with Laccase-Mediator Systems. Front. Energy Res. 2014, 2, 12. [Google Scholar] [CrossRef]
- Kunamneni, A.; Ballesteros, A.; Plou, F.J.; Alcalde, M. Fungal Laccase—A Versatile Enzyme for Biotechnological Applications. In Communicating Current Research and Educational Topics and Trends in Applied Microbiology; Mendez-Vilas, A., Ed.; FORMATEX: Badajoz, Spain, 2007; pp. 233–245. ISBN 978-84-611-9422-3. [Google Scholar]
- Bourbonnais, R.; Paice, M.G. Oxidation of Non-Phenolic Substrates. FEBS Lett. 1990, 267, 99–102. [Google Scholar] [CrossRef]
- Call, H.P.; Mücke, I. History, Overview and Application of Mediated Lignolytic Systems, Especially Lacasse-Mediator-Systems (Lignozyme®-Process). J. Biotechnol. 1997, 53, 163–202. [Google Scholar] [CrossRef]
- Wu, Y.; Teng, Y.; Li, Z.; Liao, X.; Luo, Y. Potential Role of Polycyclic Aromatic Hydrocarbons (PAHs) Oxidation by Fungal Laccase in the Remediation of an Aged Contaminated Soil. Soil Biol. Biochem. 2008, 40, 789–796. [Google Scholar] [CrossRef]
- Zeng, S.; Qin, X.; Xia, L. Degradation of the Herbicide Isoproturon by Laccase-Mediator Systems. Biochem. Eng. J. 2017, 119, 92–100. [Google Scholar] [CrossRef]
- Cañas, A.I.; Camarero, S. Laccases and Their Natural Mediators: Biotechnological Tools for Sustainable Eco-Friendly Processes. Biotechnol. Adv. 2010, 28, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Camarero, S.; Ibarra, D.; Martinez, M.J.; Martinez, A.T. Lignin-Derived Compounds as Efficient Laccase Mediators for Decolorization of Different Types of Recalcitrant Dyes. Appl. Environ. Microbiol. 2005, 71, 1775–1784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hilgers, R.; Vincken, J.-P.; Gruppen, H.; Kabel, M.A. Laccase/Mediator Systems: Their Reactivity toward Phenolic Lignin Structures. ACS Sustain. Chem. Eng. 2018, 6, 2037–2046. [Google Scholar] [CrossRef] [PubMed]
- Kurniawati, S.; Nicell, J.A. Efficacy of Mediators for Enhancing the Laccase-Catalyzed Oxidation of Aqueous Phenol. Enzyme Microb. Technol. 2007, 41, 353–361. [Google Scholar] [CrossRef]
- Mendoza, L.; Jonstrup, M.; Hatti-Kaul, R.; Mattiasson, B. Azo Dye Decolorization by a Laccase/Mediator System in a Membrane Reactor: Enzyme and Mediator Reusability. Enzyme Microb. Technol. 2011, 49, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Stoilova, I.; Krastanov, A.; Stanchev, V. Properties of Crude Laccase from Trametes versicolor Produced by Solid-Substrate Fermentation. Adv. Biosci. Biotechnol. 2010, 1, 208–215. [Google Scholar] [CrossRef]
- Mani, P.; Keshavarz, T.; Chandra, T.S.; Kyazze, G. Decolourisation of Acid orange 7 in a microbial fuel cell with a laccase-based biocathode: Influence of mitigating pH changes in the cathode chamber. Enzyme Microb. Technol. 2017, 96, 170–176. [Google Scholar] [CrossRef] [Green Version]
- Fernando, E. Treatment of Azo Dyes in Industrial Wastewater Using Microbial Fuel Cells. Ph.D. Thesis, University of Westminster, London, UK, 2014. [Google Scholar]
- Wolin, E.A.; Wolin, M.J.; Wolfe, R.S. Formation of Methane by Bacterial Extracts. J. Biol. Chem. 1963, 238, 2882–2886. [Google Scholar]
- Marsili, E.; Baron, D.B.; Shikhare, I.D.; Coursolle, D.; Gralnick, J.A.; Bond, D.R. Shewanella Secretes Flavins That Mediate Extracellular Electron Transfer. Proc. Natl. Acad. Sci. USA 2008, 105, 6–11. [Google Scholar] [CrossRef]
- Schaetzle, O.; Barrière, F.; Schröder, U. An Improved Microbial Fuel Cell with Laccase as the Oxygen Reduction Catalyst. Energy Environ. Sci. 2009, 2, 96–99. [Google Scholar] [CrossRef]
- Le Goff, A.; Holzinger, M.; Cosnier, S. Recent Progress in Oxygen-Reducing Laccase Biocathodes for Enzymatic Biofuel Cells. Cell. Mol. Life Sci. 2015, 72, 941–952. [Google Scholar] [CrossRef] [PubMed]
- Pardo, I.; Chanagá, X.; Vicente, A.I.; Alcalde, M.; Camarero, S. New Colorimetric Screening Assays for the Directed Evolution of Fungal Laccases to Improve the Conversion of Plant Biomass. BMC Biotechnol. 2013, 13, 90. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.J.; Mock, N.M.; Whitaker, B.D.; Hammond, R.W.; Nemchinov, L.; Roberts, D.P.; Aver’yanov, A.A. Characterization of Apoplast Phenolics: Invitro Oxidation of Acetosyringone Results in a Rapid and Prolonged Increase in the Redox Potential. Physiol. Mol. Plant Pathol. 2014, 86, 57–63. [Google Scholar] [CrossRef]
- Lin, H.; Su, J.; Liu, Y.; Yang, L. Catalytic Conversion of Lignocellulosic Biomass to Value-Added Organic Acids in Aqueous Media. In Application of Hydrothermal Reactions to Biomass Conversion. Green Chemistry and Sustainable Technology; Jin, F., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; p. 109. [Google Scholar]
- Volkova, N.; Ibrahim, V.; Hatti-Kaul, R. Laccase Catalysed Oxidation of Syringic Acid: Calorimetric Determination of Kinetic Parameters. Enzyme Microb. Technol. 2012, 50, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Bai, R.; Zhang, Y.; Wang, Q.; Fan, X.; Yuan, J.; Cui, L.; Wang, P. Laccase-Catalyzed Oxidative Polymerization of Phenolic Compounds. Appl. Biochem. Biotechnol. 2013, 171, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
- Lahtinen, M.; Kruus, K.; Heinonen, P.; Sipilam, J. On the reactions of two fungal laccases differing in their redox potential with lignin model compounds: Products and their rates of formation. J. Agric. Food Chem. 2009, 57, 8357–8365. [Google Scholar] [CrossRef] [PubMed]
- Martorana, A.; Sorace, L.; Boer, H.; Vazquez-Duhalt, R.; Basosi, R.; Baratto, M.C. A Spectroscopic Characterization of a Phenolic Natural Mediator in the Laccase Biocatalytic Reaction. J. Mol. Catal. B Enzym. 2013, 97, 203–208. [Google Scholar] [CrossRef]
- Bourbonnais, R.; Leech, D.; Paice, M. Electrochemical Analysis of the Interactions of Laccase Mediators with Lignin Model Compounds. Biochim. Biophys. Acta 1998, 1379, 381–390. [Google Scholar] [CrossRef]










© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mani, P.; Fidal Kumar, V.T.; Keshavarz, T.; Chandra, T.S.; Kyazze, G. The Role of Natural Laccase Redox Mediators in Simultaneous Dye Decolorization and Power Production in Microbial Fuel Cells. Energies 2018, 11, 3455. https://doi.org/10.3390/en11123455
Mani P, Fidal Kumar VT, Keshavarz T, Chandra TS, Kyazze G. The Role of Natural Laccase Redox Mediators in Simultaneous Dye Decolorization and Power Production in Microbial Fuel Cells. Energies. 2018; 11(12):3455. https://doi.org/10.3390/en11123455
Chicago/Turabian StyleMani, Priyadharshini, Vallam Thodi Fidal Kumar, Taj Keshavarz, T. Sainathan Chandra, and Godfrey Kyazze. 2018. "The Role of Natural Laccase Redox Mediators in Simultaneous Dye Decolorization and Power Production in Microbial Fuel Cells" Energies 11, no. 12: 3455. https://doi.org/10.3390/en11123455
APA StyleMani, P., Fidal Kumar, V. T., Keshavarz, T., Chandra, T. S., & Kyazze, G. (2018). The Role of Natural Laccase Redox Mediators in Simultaneous Dye Decolorization and Power Production in Microbial Fuel Cells. Energies, 11(12), 3455. https://doi.org/10.3390/en11123455