Interaction of Liquid Droplets in Gas and Vapor Flows
Abstract
:1. Introduction
2. Experimental Approach
2.1. Experimental Setup and Methods
2.2. Factors under Investigation
- Droplet sizes. In typical aerosol technologies, typical droplet sizes can change severalfold within the main range, from 0.1 to 5 mm. So, it is reasonable to vary the Rd1 and Rd2 ratio values from 0.1 to 2.
- Relative velocity of droplets. Droplet collisions in aerosols in one plane are most common. Under such conditions, droplet motion vectors are normally co-directional or opposite. Calculating the relative velocity of motion becomes much simpler, as it is rational to take into account the difference or the sum of motion velocities of two droplets. In the dimensionless processing of experimental results, either the absolute motion velocity of one of the droplets or a typical mean value for the variable range of droplet velocity can be used as a scale coefficient. Considering different schemes of droplet interaction, it appears sensible to vary the dimensionless relative velocity of a droplet in the range between 0 and 10 for real-life aerosols.
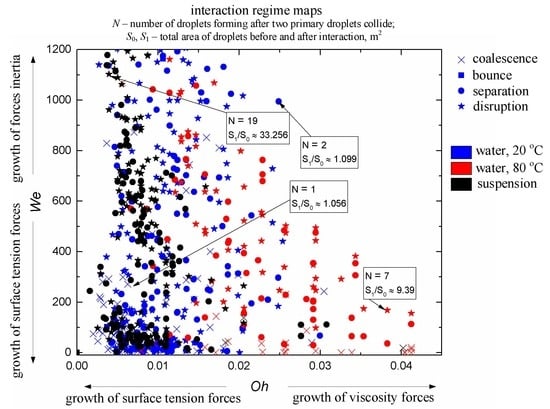
- The Weber number, characterizing the ratio of inertia to surface tension. This parameter is frequently used to describe the influence of droplet velocities and dimensions on their interaction parameters. According to the experimental studies of the last 50 years cited in the review papers [33,43,44], the We number can be found to range from 0 to 1000. This is the range, in which four main interaction modes are realized: bounce, separation, coalescence, and disruption.
- Impact (interaction) angle. The angular parameter of collision is calculated as a cosine of the interaction angle; the latter is defined as the angle between the relative velocity vector of droplets and their contact line [32] or between the trajectories, [34,43] notes that using this or that angular parameter of interaction is arguable. At a first approximation, both approaches can be used with varying the angle between droplet trajectories in the widest range, i.e., from 0° to 90°. Based on the data from [34,43], we can recalculate the experimental results to fit the techniques in [32].
- Droplet concentration in an aerosol. This parameter affects the frequency of droplet collisions. It is interesting to study its influence on the droplet interaction mode and typical outcomes. The variation range of γd can be quite wide to characterize all the possible atomization conditions. Since the volume of each droplet as an aerosol element is incomparably smaller than that of power plant chambers, the relative concentration range should be chosen in accordance with the mean droplet size in the aerosol.
- Temperature range in the droplet collision area, which depends on the temperature of both the gas medium (Ta) and liquid droplets (Td). In accordance with the real-life gas-vapor-droplet applications, the most typical variation ranges for these parameters are up to 1000 °C for Ta and up to 100 °C for Td. Using an air heater and compressor, as well as a flue gas generation system, similarly to the method in [20,21], we can vary these parameters in wide ranges.
- Turbulent, transition, or laminar flow regimes of the carrier medium. Typical spraying and irrigation systems feature different motion regimes of the aerosol in general and single droplets as its elements. Thus, the Reynolds number for the carrier gas medium can vary from several dozen to several thousand. It is important to vary the characteristic values for such a wide Re range. For instance, it is reasonable to consider the range up to 3000 for typical systems using flows based on flue gases.
- Dimensionless parameters of droplet interaction. Traditionally, two types of interaction parameters are used: linear (B) and angular (β). The angular parameter was calculated by the formula β = cos(αd), whereas the linear one took into account the impact parameter B = b/(Rd1 + Rd2). The typical range of varying these parameters is 0 to 1 [43].
- Heterogeneity in droplet composition. In this case, we draw up interaction parameter maps using the Ohnesorge, Laplace, Reynolds, and capillary numbers. The characteristic variation ranges of Oh, La, Ca, and Re in such maps can differ, but they are generally comparable to the known data for water. Thus, we can evaluate the effect of viscosity, surface tension, density, and other properties of liquids on droplet transformation characteristics. The analysis of [33,43,44] shows that there is a lot of data for droplets of homogeneous liquids, solutions, and emulsions. So, it is slurry compositions that attract attention as the object of research. The influence of concentration and size of solid particles on lamination and other processes is traditionally extensively discussed (an overview of this issue is presented in [42]). In this study, we selected the mean values of these parameters in accordance with the concepts in [42] (to provide for a further assessment for different technologies, in which these parameters vary in wide ranges, sometimes severalfold): the graphite particles used were 40–50 µm in size, and their relative mass fraction was 5%.
- Ratios of initial surface areas of interacting droplets and their roles: target or projectile. The results of processing the experimental data on liquid droplet interaction are often generalized with no emphasis on the droplet role. The experiments [20,21] have shown that pre-collision projectiles and targets can differ not only in size and velocity but also in shape. This factor should be taken into account when data is generalized, and especially when the outcomes of droplet collision are analyzed. It makes sense to determine the average number of new droplets N and the ratio S1/S0.
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature and Units
| b | distance between droplet centers of mass, mm; |
| B | dimensionless linear parameter of interaction; |
| Ca | capillary number; |
| La | Laplace number; |
| l | distance, m; |
| n1, n2, n3, n4 | the number of collisions corresponding to coalescence, separation, disruption, and bounce; |
| N | number of droplets forming after two primary droplets collide; |
| Oh | Ohnesorge number; |
| P1, P2, P3, P4 | relative frequency of occurrence of coalescence, separation, disruption, and bounce; |
| Rd | radii of droplets, mm; |
| Rd1, Rd2 | radii of the first and second droplets, mm; |
| Re | Reynolds number; |
| r | segment size, m; |
| s | scale factor, mm/pix; |
| S0 | total area of droplets before interaction, m2; |
| S1 | total area of satellite droplets after the primary ones collide, m2; |
| Sm | frontal cross-sectional area, mm2; |
| t | time, s; |
| T | temperature, °C; |
| Ta, Td | temperature of gases and droplet, °C; |
| Ud0 | scale of velocity used in dimensionless processing of experimental results (Ud0 = 1 m/s), m/s; |
| Ud | velocity of droplet, m/s; |
| Ud1, Ud2 | velocities of the first and second droplets, m/s; |
| Ug | carrier gas medium velocity, m/s; |
| Urel | relative droplet velocity, m/s; |
| We | Weber number. |
| Greek symbols | |
| αd | impact angle, °; |
| β | dimensionless angular parameter of interaction; |
| γ0 | initial droplet concentration (L/m3); |
| γd | concentration of droplets in aerosol cloud (L/m3); |
| γmin | scale (minimum) of droplet concentration (L/m3); |
| δl | systematic errors of the distance, m; |
| δt | systematic errors of the time, s; |
| δαd | systematic errors of the angle, °; |
| µ | dynamic viscosity, Pa∙s; |
| ρ | density; kg/m3; |
| σ | surface tension, N/m. |
References
- Castanet, G.; Labergue, A.; Lemoine, F. Internal temperature distributions of interacting and vaporizing droplets. Int. J. Therm. Sci. 2011, 50, 1181–1190. [Google Scholar] [CrossRef] [Green Version]
- Yan, L.; Li, H.; Li, L.; Wu, C. Bubble behavior of flow boiling in horizontal rectangular channels with inclined ribs. Int. J. Heat Mass Transf. 2014, 75, 514–522. [Google Scholar] [CrossRef]
- Xu, Q.; Guo, L.; Zou, S.; Chen, J.; Zhang, X. Experimental study on direct contact condensation of stable steam jet in water flow in a vertical pipe. Int. J. Heat Mass Transf. 2013, 66, 808–817. [Google Scholar] [CrossRef]
- Augusto, C.M.; Ribeiro, J.B.; Gaspar, A.R.; Costa, J.J. Low-pressure-vaporization of free water – Characterization of the boiling regimes. Int. J. Therm. Sci. 2014, 77, 19–26. [Google Scholar] [CrossRef]
- Willert, C.E. Assessment of camera models for use in planar velocimetry calibration. Exp. Fluids 2006, 41, 135–143. [Google Scholar] [CrossRef]
- Corvaro, F.; Paroncini, M.; Sotte, M. Experimental PIV and interferometric analysis of natural convection in a square enclosure with partially active hot and cold walls. Int. J. Therm. Sci. 2011, 50, 1629–1638. [Google Scholar] [CrossRef]
- Young, C.N.; Johnson, D.A.; Weckman, E.J. A model-based validation framework for PIV and PTV. Exp. Fluids 2004, 36, 23–35. [Google Scholar] [CrossRef]
- Hadad, T.; Gurka, R. Effects of particle size, concentration and surface coating on turbulent flow properties obtained using PIV/PTV. Exp. Therm. Fluid Sci. 2013, 45, 203–212. [Google Scholar] [CrossRef]
- Stepanov, E.Y.; Maslov, V.P.; Zakharov, D.L. A stereo PIV system for measuring the velocity vector in complex gas flows. Meas. Tech. 2009, 52, 626–631. [Google Scholar] [CrossRef]
- Legrand, M.; Nogueira, J.; Lecuona, A.; Nauri, S.; Rodríguez, P.A. Atmospheric low swirl burner flow characterization with stereo PIV. Exp. Fluids 2010, 48, 901–913. [Google Scholar] [CrossRef]
- Damaschke, N.; Nobach, H.; Tropea, C. Optical limits of particle concentration for multi-dimensional particle sizing techniques in fluid mechanics. Exp. Fluids 2002, 32, 143–152. [Google Scholar]
- Bilsky, A.V.; Lozhkin, Y.A.; Markovich, D.M. Interferometric technique for measurement of droplet diameter. Thermophys. Aeromechanics 2011, 18. [Google Scholar] [CrossRef]
- Akhmetbekov, Y.K.; Alekseenko, S.V.; Dulin, V.M.; Markovich, D.M.; Pervunin, K.S. Planar fluorescence for round bubble imaging and its application for the study of an axisymmetric two-phase jet. Exp. Fluids 2010, 48, 615–629. [Google Scholar] [CrossRef]
- Dulin, V.; Markovich, D.; Pervunin, K. The optical principles of PFBI approach. AIP Conf. Proc. 2012, 1428, 217–224. [Google Scholar]
- Khalili, I.; Dufaud, O.; Poupeau, M.; Cuervo-Rodriguez, N.; Perrin, L. Ignition sensitivity of gas–vapor/dust hybrid mixtures. Powder Technol. 2012, 217, 199–206. [Google Scholar] [CrossRef]
- Liu, B.; Cai, J.; Huai, X. Heat transfer with the growth and collapse of cavitation bubble between two parallel heated walls. Int. J. Heat Mass Transf. 2014, 78, 830–838. [Google Scholar] [CrossRef]
- Barai, N.; Mandal, N. Breakup versus coalescence of closely packed fluid drops in simple shear flows. Int. J. Multiph. Flow 2019, 1–15. [Google Scholar] [CrossRef]
- Holz, S.; Braun, S.; Chaussonnet, G.; Koch, R.; Bauer, H.-J. Close nozzle spray characteristics of a prefilming airblast atomizer. Energies 2019, 12, 2835. [Google Scholar] [CrossRef]
- Eggers, J.; Villermaux, E. Physics of liquid jets. Rep. Prog. Phys. 2008, 71. [Google Scholar] [CrossRef]
- Volkov, R.S.; Kuznetsov, G.V.; Strizhak, P.A. Statistical analysis of consequences of collisions between two water droplets upon their motion in a high-temperature gas flow. Tech. Phys. Lett. 2015, 41, 840–843. [Google Scholar] [CrossRef]
- Antonov, D.V.; Volkov, R.S.; Kuznetsov, G.V.; Strizhak, P.A. Experimental Study of the Effects of Collision of Water Droplets in a Flow of High-Temperature Gases. J. Eng. Phys. Thermophys. 2016, 89, 100–111. [Google Scholar] [CrossRef]
- Vysokomornaya, O.V.; Kuznetsov, G.V.; Strizhak, P.A. Evaporation of Water Droplets in a High-Temperature Gaseous Medium. J. Eng. Phys. Thermophys. 2016, 89, 141–151. [Google Scholar] [CrossRef]
- Nishad, K.; Sadiki, A.; Janicka, J. Numerical Investigation of AdBlue Droplet Evaporation and Thermal Decomposition in the Context of NOx-SCR Using a Multi-Component Evaporation Model. Energies 2018, 11, 222. [Google Scholar] [CrossRef]
- Fan, X.; Liu, C.; Mu, Y.; Wang, K.; Wang, Y.; Xu, G. Experimental investigations of flow field and atomization field characteristics of pre-filming air-blast atomizers. Energies 2019, 12, 2800. [Google Scholar] [CrossRef]
- Arkhipov, V.A.; Ratanov, G.S.; Trofimov, V.F. Experimental investigation of the interaction of colliding droplets. J. Appl. Mech. Tech. Phys. 1978, 19, 201–204. [Google Scholar] [CrossRef]
- Arkhipov, V.A.; Vasenin, I.M.; Trofimov, V.F. Stability of colliding drops of ideal liquid. J. Appl. Mech. Tech. Phys. 1983, 24, 371–373. [Google Scholar] [CrossRef]
- Wierzba, A. Deformation and breakup of liquid drops in a gas stream at nearly critical Weber numbers. Exp. Fluids 1990, 9, 59–64. [Google Scholar] [CrossRef]
- Shraiber, A.A.; Podvysotsky, A.M.; Dubrovsky, V.V. Deformation and breakup of drops by aerodynamic forces. At. Sprays 1996, 6, 667–692. [Google Scholar] [CrossRef]
- Arkhipov, V.A.; Vasenin, I.M.; Trofimov, V.F.; Usanina, A.S. Stability of dispersed-particle shape at small Reynolds numbers. Fluid Dyn. 2013, 48, 143–150. [Google Scholar] [CrossRef]
- Bjørge, J.S.; Bjørkheim, S.A.; Metallinou, M.-M.; Log, T.; Frette, Ø. Influence of acetone and sodium chloride additives on cooling efficiency of water droplets impinging onto hot metal surfaces. Energies 2019, 12, 2358. [Google Scholar] [CrossRef]
- Teodori, E.; Pontes, P.; Moita, A.; Georgoulas, A.; Marengo, M.; Moreira, A. Sensible heat transfer during droplet cooling: Experimental and numerical analysis. Energies 2017, 10, 790. [Google Scholar] [CrossRef]
- Pawar, S.K.; Henrikson, F.; Finotello, G.; Padding, J.T.; Deen, N.G.; Jongsma, A.; Innings, F.; Kuipers, J.A.M.H. An experimental study of droplet-particle collisions. Powder Technol. 2016, 300, 157–163. [Google Scholar] [CrossRef] [Green Version]
- Charalampous, G.; Hardalupas, Y. Collisions of droplets on spherical particles. Phys. Fluids 2017, 29, 103305. [Google Scholar] [CrossRef] [Green Version]
- Kan, H.; Nakamura, H.; Watano, S. Effect of collision angle on particle-particle adhesion of colliding particles through liquid droplet. Adv. Powder Technol. 2018, 29, 1317–1322. [Google Scholar] [CrossRef]
- Chen, S.; Bartello, P.; Yau, M.K.; Vaillancourt, P.A.; Zwijsen, K. Cloud droplet collisions in turbulent environment: Collision statistics and parameterization. J. Atmos. Sci. 2016, 73, 621–636. [Google Scholar] [CrossRef]
- Williams, Y.O.; Roas-Escalona, N.; Rodríguez-Lopez, G.; Villa-Torrealba, A.; Toro-Mendoza, J. Modeling droplet coalescence kinetics in microfluidic devices using population balances. Chem. Eng. Sci. 2019, 201, 475–483. [Google Scholar] [CrossRef]
- Knothe, G.; Steidley, K.R. Kinematic viscosity of biodiesel components (fatty acid alkyl esters) and related compounds at low temperatures. Fuel 2007, 86, 2560–2567. [Google Scholar] [CrossRef]
- Lunkenheimer, K.; Wantke, K.D. On the applicability of the du Nouy (ring) tensiometer method for the determination of surface tensions of surfactant solutions. J. Colloid Interface Sci. 1978, 66, 579–581. [Google Scholar] [CrossRef]
- Finotello, G.; Kooiman, R.F.; Padding, J.T.; Buist, K.A.; Jongsma, A.; Innings, F.; Kuipers, J.A.M. The dynamics of milk droplet--droplet collisions. Exp. Fluids 2018, 59, 17. [Google Scholar] [CrossRef]
- Finotello, G.; De, S.; Vrouwenvelder, J.C.R.; Padding, J.T.; Buist, K.A.; Jongsma, A.; Innings, F.; Kuipers, J.A.M. Experimental investigation of non-Newtonian droplet collisions: The role of extensional viscosity. Exp. Fluids 2018, 59, 113. [Google Scholar] [CrossRef]
- Pan, K.-L.; Tseng, Y.-H.; Chen, J.-C.; Huang, K.-L.; Wang, C.-H.; Lai, M.-C. Controlling droplet bouncing and coalescence with surfactant. J. Fluid Mech. 2016, 799, 603–636. [Google Scholar] [CrossRef]
- Vysokomornaya, O.V.; Piskunov, M.V.; Strizhak, P.A. Breakup of heterogeneous water drop immersed in high-temperature air. Appl. Therm. Eng. 2017, 127, 1340–1345. [Google Scholar] [CrossRef]
- Orme, M. Experiments on droplet collisions, bounce, coalescence and disruption. Prog. Energy Combust. Sci. 1997, 23, 65–79. [Google Scholar] [CrossRef]
- Wang, Y.; Gratadeix, A.; Do-Quang, M.; Amberg, G. Events and conditions in droplet impact: A phase field prediction. Int. J. Multiph. Flow 2016, 87, 54–65. [Google Scholar] [CrossRef]
- Qian, J.; Law, C.K. Regimes of coalescence and separation in droplet collision. J. Fluid Mech. 1997, 331, 59–80. [Google Scholar] [CrossRef]
- Antonov, D.V.; Strizhak, P.A. Heating, evaporation, fragmentation, and breakup of multi-component liquid droplets when heated in air flow. Chem. Eng. Res. Des. 2019, 146, 22–35. [Google Scholar] [CrossRef]
- Cimpeanu, R.; Papageorgiou, D.T. Three-dimensional high speed drop impact onto solid surfaces at arbitrary angles. Int. J. Multiph. Flow 2018, 107, 192–207. [Google Scholar] [CrossRef]













| Liquid | Temperature, T (°C) | Density ρ (kg/m3) | Surface Tension, σ∙103 (N/m) | Dynamic Viscosity, µ∙106 (Pa∙s) |
|---|---|---|---|---|
| Water | 20/80 | 998/965 | 72/63 | 1004/282 |
| Slurry | 20 | 1030 | 73 | 1100 |
| Milk [39,40] | 20 | 1041 | 47 | 4300 |
| Water [32] | 20–22 | 995–1000 | 72–73 | 1000–1010 |
| No. of collision under study | 1 | … | 1012 |
| ɑd—impact angle, ° | 5 | … | 0 |
| Rd1—radius of the first droplet, mm | 0.253 | … | 0.151 |
| Rd2—radius of the first droplet, mm | 0.301 | … | 0.334 |
| Ud1—velocity of the first droplet, m/s | 1.823 | … | 2.93 |
| Ud2—velocity of the second droplet, m/s | 2.431 | … | 3.13 |
| b—distance between droplet centers of mass, mm | 0.234 | … | 0.409 |
| Interaction mode | Bounce | … | Disruption |
| No. of frame | 22 | … | 3297 |
| Shape of the first droplet | sphere | … | sphere |
| Shape of the second droplet | sphere | … | sphere |
| N—number of post-collision droplets | 2 | … | 14 |
| Type of droplet interaction | co-directional | … | head-on |
| Rd1/Rd2 | 0.841 | … | 0.452 |
| Urel—resulting velocity of droplets after interaction, m/s | 0.6 | … | 6.06 |
| B [b/(Rd1 + Rd2)] | 0.423 | … | 0.844005 |
| β [cos(ɑd)] | 0.996 | … | 1 |
| … | … | … | … |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demidovich, A.V.; Kralinova, S.S.; Tkachenko, P.P.; Shlegel, N.E.; Volkov, R.S. Interaction of Liquid Droplets in Gas and Vapor Flows. Energies 2019, 12, 4256. https://doi.org/10.3390/en12224256
Demidovich AV, Kralinova SS, Tkachenko PP, Shlegel NE, Volkov RS. Interaction of Liquid Droplets in Gas and Vapor Flows. Energies. 2019; 12(22):4256. https://doi.org/10.3390/en12224256
Chicago/Turabian StyleDemidovich, A. V., S. S. Kralinova, P. P. Tkachenko, N. E. Shlegel, and R. S. Volkov. 2019. "Interaction of Liquid Droplets in Gas and Vapor Flows" Energies 12, no. 22: 4256. https://doi.org/10.3390/en12224256
APA StyleDemidovich, A. V., Kralinova, S. S., Tkachenko, P. P., Shlegel, N. E., & Volkov, R. S. (2019). Interaction of Liquid Droplets in Gas and Vapor Flows. Energies, 12(22), 4256. https://doi.org/10.3390/en12224256