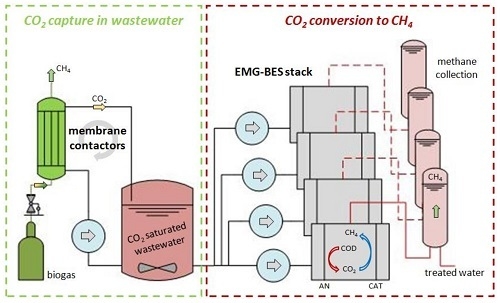
Integration of Membrane Contactors and Bioelectrochemical Systems for CO2 Conversion to CH4
Abstract
:Highlights:
- The CO2 captured in wastewater can be converted to CH4 through electromethanogenesis (Carbon Capture and Utilization, CCU).
- The process is suitable to chemically store renewable energy surplus as CH4 (Power to fuel).
- Membrane contactors for CO2 capture and bioelectrochemical systems (BES) for electromethanogenesis were successfully integrated for the first time.
- A stack of BES, fed with real municipal wastewater, was long-term tested under different electric configurations.
- A production of 4.6 L CH4 m−2 d−1 was achieved while storing 65% of applied electric energy.
- Carbon conversion efficiency was limited depending on CO2 amount injected in wastewater.
1. Introduction
2. Materials and Methods
2.1. CO2 Capture into Wastewater by Membrane Contactors
2.2. EMG-BES Construction, Operation and Characterization
3. Results and Discussion
3.1. CO2 Capture in Wastewater through Membrane Contactors
3.2. Operation of Individual EMG-BES at Constant Voltage and Synthetic Feeding
3.3. Operation of Individual EMG-BES at Constant Voltage and Municipal Wastewater Feeding
3.4. Operation of Series-Stacked EMG-BES with Municipal Wastewater Feeding
3.5. Carbon Conversion and Energy Storage Efficiency of EMG-BES Technology
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Test | pH0 | pHfin | EC0 (mS cm−1) | ECfin (mS cm−1) | TC0 (mg L−1) | TCfin (mg L−1) | Efficiency loss (%) |
|---|---|---|---|---|---|---|---|
| Test 1 | 13.5 | 10.4 | 16.0 | 7.9 | 200.5 | 960.5 | clean |
| Test 2 | 13.9 | 10.9 | 31.3 | 21.5 | 291.3 | 689.7 | 28 |
| Test 3 | 13.7 | 11.7 | 15.5 | 10.3 | 138.2 | 552.4 | 42 |
| Test 4 | 13.43 | 9.94 | 13.68 | 7.72 | 249.6 | 933.5 | clean |
| Parameter | Value |
|---|---|
| pH | 7.4 ± 0.2 |
| Conductivity (mS cm−1) | 7.7 ± 0.1 |
| COD (g O2 L−1) | 22.8 ± 1.9 |
| BOD5 (g O2 L−1) | 1.054 |
| NTOT (g L−1) | 0.739 ± 0.396 |
| TSS (g L−1) | 22.9 ± 0.3 |
| VSS (g L−1) | 14.1 ± 1.4 |
| Component | Concentration (mg L−1) |
|---|---|
| FeCl3 | 650 |
| ZnCl2 | 70 |
| H3BO3 | 6 |
| NiCl2 · 6H2O | 24 |
| Na2MO4 · 2H2O | 36 |
| CoCl2 · 6H2O | 238 |
| CaCl2 · 2H2O | 87 |
| CuSO4 · 5H2O | 2.9 |
| MnSO4 · H2O | 85.3 |
| Parameter | (i) at WWTP | (ii) after CO2 Capture* | (iii) after EMG-BES |
|---|---|---|---|
| pH | 7.5 ± 0.2 | 7.7 ± 0.2 | 8.3 ± 0.3 |
| Conductivity (mS cm−1) | 2.3 ± 0.1 | 11.5 ± 3.1 | 10.8 ± 2.2 |
| COD (mg O2 L−1) | 421.4 ± 34.1 | 1346.9 ± 97.6 | 218.6 ± 7.0 |
| BOD5 (mg O2 L−1) | 240.9 ± 20.7 | - | - |
| NTOT (mg L−1) | 60.6 ± 3.9 | - | - |
| Cycle | Electric Parameters | Liquid Phase Parameters | Gas Phase Parameters | Efficiency Parameters | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N - | Day* - | Current mA cm−2 | Ecat V vs SHE | pHOUT - | ECOUT mS cm−1 | OLR Kg COD m−3 d−1 | ηCOD % | O2 % | N2 % | CH4 % | CO2 % | CH4 rate L m−2 d−1 | Air cont. mL d−1 | Energy kWh m−3CH4 | CEan % | CEcat % | ηCONV % |
| 18 | 127 | 0.054 | −0.814 | 8.48 | 10.1 | 0.433 | 91 | 2.5 | 70.7 | 12.0 | 0.8 | 0.176 | 22.6 | 51.5 | 23.4 | 13.0 | 0.8 |
| 26 | 183 | 0.071 | −0.789 | - | - | 0.610 | - | 1.3 | 59.5 | 35.1 | 1.6 | 0.895 | 33.0 | 13.3 | - | 50.3 | 2.4 |
| 33 | 235 | 0.012 | - | 8.34 | 9.97 | 0.554 | 96 | 2.2 | 75.3 | 21.1 | 1.9 | 0.330 | 25.6 | 6.1 | 3.8 | 109.7 | 1.0 |
| 34 | 239 | 0.009 | - | 8.11 | 9.61 | 0.757 | 92 | 1.8 | 72.0 | 24.7 | 1.2 | 0.437 | 27.7 | 3.5 | 2.2 | 193.7 | 1.2 |
| 36 | 266 | 0.009 | −0.431 | 8.44 | 8.51 | 0.891 | 93 | 8.7 | 62.4 | 30.1 | 0.8 | 0.815 | 36.8 | 1.9 | 1.9 | 361.2 | 1.9 |
| Cycle | Electric Parameters | Liquid Phase Parameters | Gas Phase Parameters | Efficiency Parameters | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N - | Day* - | Current mA cm−2 | Ecat V vs SHE | pHOUT - | ECOUT mS cm−1 | OLR Kg COD m−3 d−1 | ηCOD % | O2 % | N2 % | CH4 % | CO2 % | CH4 rate L m−2 d−1 | Air cont. mL d−1 | Energy kWh m−3CH4 | CEan % | CEcat % | ηCONV % |
| 18 | 127 | 0.043 | −0.886 | 8.5 | 10.16 | 0.577 | 92 | 3.8 | 68.9 | 13.3 | 0.9 | 0.137 | 15.4 | 52.7 | 13.8 | 12.7 | 0.5 |
| 26 | 183 | 0.048 | −0.876 | - | - | 0.718 | - | 1.9 | 58 | 35 | 2.1 | 0.892 | 32.2 | 9.0 | - | 74.1 | 2.6 |
| 33 | 235 | 0.009 | - | 8.23 | 9.26 | 0.718 | 92 | 5.7 | 83.3 | 7.9 | 0.3 | 0.146 | 33.5 | 10.4 | 2.3 | 64.7 | 0.5 |
| 34 | 239 | 0.012 | - | 8.13 | 10.5 | 0.718 | 93 | 2.2 | 70.9 | 24.1 | 1.9 | 1.281 | 82.0 | 1.6 | 3.1 | 425.8 | 4.4 |
| 36 | 266 | 0.006 | −0.426 | 8.47 | 8.6 | 0.669 | 94 | 6.5 | 63.9 | 30.6 | 1.1 | 0.978 | 44.5 | 1.0 | 1.6 | 650.1 | 3.0 |
| Cycle | Electric Parameters | Liquid Phase Parameters | Gas Phase Parameters | Efficiency Parameters | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N - | Day* - | Current mA cm−2 | Ecat V vs SHE | pHOUT - | ECOUT mS cm−1 | OLR Kg COD m−3 d−1 | ηCOD % | O2 % | N2 % | CH4 % | CO2 % | CH4 rate L m−2 d−1 | Air cont. mL d−1 | Energy kWh m−3CH4 | CEan % | CEcat % | ηCONV % |
| 18 | 127 | 0.075 | −0.825 | 8.57 | 10.12 | 0.511 | 89 | 2.5 | 64.4 | 19.1 | 0.5 | 0.418 | 30.7 | 30.1 | 28.1 | 22.2 | 1.7 |
| 26 | 183 | 0.026 | −0.807 | - | - | 0.423 | - | 1.9 | 71.2 | 17.7 | 0.3 | 0.487 | 42.6 | 9.0 | - | 74.7 | 2.4 |
| 33 | 235 | 0.012 | - | 8.33 | 9.83 | 0.423 | 96 | 2.4 | 78.1 | 17.7 | 1.7 | 0.589 | 56.6 | 3.4 | 5.0 | 195.8 | 1.4 |
| 34 | 239 | 0.010 | - | 8.13 | 10.52 | 0.423 | 97 | 2.4 | 66.6 | 29.7 | 1.5 | 0.875 | 42.7 | 1.9 | 4.2 | 349.0 | 2.9 |
| 36 | 266 | 0.007 | −0.439 | 8.19 | 10.44 | 0.655 | 96 | 1.9 | 58.3 | 41.9 | 2.0 | 1.786 | 54.1 | 0.7 | 1.9 | 1018 | 5.6 |
| Cycle | Electric Parameters | Liquid Phase Parameters | Gas Phase Parameters | Efficiency Parameters | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N - | Day* - | Current mA cm−2 | Ecat V vs SHE | pHOUT - | ECOUT mS cm−1 | OLR Kg COD m−3 d−1 | ηCOD % | O2 % | N2 % | CH4 % | CO2 % | CH4 rate L m−2 d−1 | Air cont. mL d−1 | Energy kWh m−3CH4 | CEan % | CEcat % | ηCONV % |
| 18 | 127 | 0.062 | −0.806 | 8.54 | 9.91 | 0.511 | 93 | 2.1 | 59.2 | 27.1 | 0.7 | 0.358 | 17.0 | 29.1 | 22.3 | 23.0 | 1.5 |
| 26 | 183 | 0.017 | −0.426 | - | - | 0.728 | - | 3 | 56.2 | 32.5 | 4.2 | 0.868 | 32.7 | 3.3 | - | 203.6 | 2.5 |
| 33 | 235 | 0.013 | - | 8.36 | 8.87 | 0.728 | 97 | 3.2 | 77.5 | 18.5 | 0.8 | 0.158 | 14.4 | 13.8 | 3.1 | 48.5 | 0.5 |
| 34 | 239 | 0.011 | - | 8.04 | 10.4 | 0.728 | 92 | 2.0 | 63.4 | 33.4 | 1.9 | 1.673 | 69.1 | 1.1 | 2.8 | 606.6 | 6.2 |
| 36 | 266 | 0.009 | −0.452 | 8.46 | 8.93 | 0.813 | 93 | 2.9 | 59.7 | 38.7 | 1.6 | 1.348 | 45.3 | 1.1 | 2.0 | 597.4 | 3.4 |
| Cycle | Electric Parameters | Liquid Phase Parameters | Gas Phase Parameters | Efficiency Parameters | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N - | Day* - | Current mA cm−2 | Ecat V vs SHE | pHOUT - | ECOUT mS cm−1 | OLR Kg COD m–3 d−1 | ηCOD % | O2 % | N2 % | CH4 % | CO2 % | CH4 rate L m−2 d−1 | Air cont. mL d−1 | Energy kWh m−3CH4 | CEan % | CEcat % | ηCONV % |
| 18 | 127 | 0.049 | −0.776 | 8.48 | 10.10 | 0.564 | 93 | 3.9 | 67.4 | 7.9 | 0.8 | 0.087 | 16.2 | 94.6 | 15.9 | 7.1 | 0.3 |
| 26 | 183 | 0.020 | −0.386 | - | - | 0.393 | - | 2.3 | 59.0 | 31.3 | 2.0 | 0.580 | 23.8 | 5.8 | - | 115.7 | 3.1 |
| 34 | 239 | 0.015 | - | 8.11 | 9.61 | 0.777 | 93 | 3.0 | 69.2 | 29.9 | 1.2 | 1.155 | 58.2 | 2.2 | 3.5 | 307.1 | 3.4 |
| 36 | 266 | 0.009 | −0.418 | 8.44 | 8.51 | 0.813 | 97 | 1.8 | 60.4 | 38.2 | 2.0 | 0.778 | 26.8 | 1.9 | 1.9 | 344.8 | 2.0 |
| 39 | 288 | 0.005 | - | - | - | 0.905 | 98 | 0.0 | 39.6 | 45.7 | 1.8 | 1.455 | 27.4 | 0.6 | 1.0 | 1161 | 3.3 |
| 49 | 351 | 0.057 | - | 8.40 | 10.80 | 0.560 | 87 | 0.6 | 70.4 | 17.6 | 0.5 | 0.341 | 29.7 | 28.1 | 20.0 | 23.9 | 0.7 |
| 50 | 358 | 0.092 | - | 8.42 | 11.04 | 0.630 | 83 | 0.9 | 55.0 | 35.2 | 1.1 | 0.827 | 28.1 | 18.7 | 30.0 | 35.9 | 1.8 |
| 51 | 365 | 0.092 | - | 8.49 | 10.26 | 0.455 | 84 | 0.4 | 65.7 | 35.8 | 1.2 | 0.843 | 33.7 | 18.3 | 41.1 | 36.5 | 2.2 |
| 52 | 373 | 0.083 | - | 8.26 | 9.03 | 0.530 | 76 | 0.3 | 68.5 | 33.4 | 1.6 | 0.830 | 37.1 | 16.8 | 35.1 | 39.9 | 2.7 |
| 53 | 379 | 0.056 | - | 8.53 | 8.26 | 0.631 | 78 | 0.0 | 67.6 | 37.0 | 1.9 | 1.167 | 46.4 | 8.1 | 19.4 | 83.1 | 3.7 |
References
- International Energy Agency. Energy Technology Perspectives 2010; International Energy Agency: Paris, France, 2010. [Google Scholar]
- European Commission. European Energy Roadmap 2050; European Commission: Brussels, Belgium, 2011. [Google Scholar]
- World Energy Council World Energy Resources-E-Storage. Available online: https://www.worldenergy.org/wp-content/uploads/2017/03/WEResources_E-storage_2016.pdf. (accessed on 22 January 2019).
- Directorate-General for Research and Innovation (European Commission). Novel Carbon Capture and Utilisation Technologies; European Commission: Bruxells, Belgium, 2017. [Google Scholar]
- Bailera, M.; Lisbona, P.; Romeo, L.M.; Espatolero, S. Power to Gas projects review: Lab, pilot and demo plants for storing renewable energy and CO2. Renew. Sustain. Energy Rev. 2017, 69, 292–312. [Google Scholar] [CrossRef]
- Batlle-Vilanova, P.; Puig, S.; Gonzalez-Olmos, R.; Vilajeliu-Pons, A.; Balaguer, M.D.; Colprim, J. Deciphering the electron transfer mechanisms for biogas upgrading to biomethane within a mixed culture biocathode. RSC Adv. 2015, 5, 52243–52251. [Google Scholar] [CrossRef] [Green Version]
- Götz, M.; Koch, A.M.; Graf, F. State of the Art and Perspectives of CO2 Methanation Process Concepts for Power-to-Gas Applications. In Proceedings of the International Gas Union Research Conference, Copenhagen, Denmark, 17–19 September 2014. [Google Scholar]
- Cheng, S.; Xing, D.; Call, D.F.; Logan, B.E. Direct Biological Conversion of Electrical Current into Methane by Electromethanogenesis. Environ. Sci. Technol. 2009, 43, 3953–3958. [Google Scholar] [CrossRef]
- Blasco-Gómez, R.; Batlle-Vilanova, P.; Villano, M.; Balaguer, M.; Colprim, J.; Puig, S. On the Edge of Research and Technological Application: A Critical Review of Electromethanogenesis. Int. J. Mol. Sci. 2017, 18, 874. [Google Scholar] [CrossRef]
- Choi, K.-S.; Kondaveeti, S.; Min, B. Bioelectrochemical methane (CH4) production in anaerobic digestion at different supplemental voltages. Bioresour. Technol. 2017, 245, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Bajracharya, S.; Sharma, M.; Mohanakrishna, G.; Dominguez Benneton, X.; Strik, D.P.B.T.B.; Sarma, P.M.; Pant, D. An overview on emerging bioelectrochemical systems (BESs): Technology for sustainable electricity, waste remediation, resource recovery, chemical production and beyond. Renew. Energy 2016, 98, 153–170. [Google Scholar] [CrossRef]
- Rabaey, K.; Rozendal, R.A. Microbial electrosynthesis—Revisiting the electrical route for microbial production. Nat. Rev. Microbiol. 2010, 8, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Molenaar, S.D.; Mol, A.R.; Sleutels, T.H.J.A.; Ter Heijne, A.; Buisman, C.J.N. Microbial Rechargeable Battery: Energy Storage and Recovery through Acetate. Environ. Sci. Technol. Lett. 2016, 3, 144–149. [Google Scholar] [CrossRef]
- Van Eerten-Jansen, M.C.A.A.; Jansen, N.C.; Plugge, C.M.; de Wilde, V.; Buisman, C.J.N.; ter Heijne, A. Analysis of the mechanisms of bioelectrochemical methane production by mixed cultures. J. Chem. Technol. Biotechnol. 2015, 90, 963–970. [Google Scholar] [CrossRef]
- Villano, M.; Aulenta, F.; Ciucci, C.; Ferri, T.; Giuliano, A.; Majone, M. Bioelectrochemical reduction of CO2 to CH4 via direct and indirect extracellular electron transfer by a hydrogenophilic methanogenic culture. Bioresour. Technol. 2010, 101, 3085–3090. [Google Scholar] [CrossRef]
- Jiang, Y.; Su, M.; Zhang, Y.; Zhan, G.; Tao, Y.; Li, D. Bioelectrochemical systems for simultaneously production of methane and acetate from carbon dioxide at relatively high rate. Int. J. Hydrog. Energy 2013, 38, 3497–3502. [Google Scholar] [CrossRef] [Green Version]
- Zhen, G.; Zheng, S.; Lu, X.; Zhu, X.; Mei, J.; Kobayashi, T.; Xu, K.; Li, Y.-Y.; Zhao, Y. A comprehensive comparison of five different carbon-based cathode materials in CO2 electromethanogenesis: Long-term performance, cell-electrode contact behaviors and extracellular electron transfer pathways. Bioresour. Technol. 2018, 266, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Baek, G.; Kim, J.; Lee, S.; Lee, C. Development of biocathode during repeated cycles of bioelectrochemical conversion of carbon dioxide to methane. Bioresour. Technol. 2017, 241, 1201–1207. [Google Scholar] [CrossRef]
- Dykstra, C.M.; Pavlostathis, S.G. Methanogenic Biocathode Microbial Community Development and the Role of Bacteria. Environ. Sci. Technol. 2017, 51, 5306–5316. [Google Scholar] [CrossRef] [PubMed]
- Siegert, M.; Yates, M.D.; Spormann, A.M.; Logan, B.E. Methanobacterium Dominates Biocathodic Archaeal Communities in Methanogenic Microbial Electrolysis Cells. ACS Sustain. Chem. Eng. 2015, 3, 1668–1676. [Google Scholar] [CrossRef]
- Yang, H.-Y.; Bao, B.-L.; Liu, J.; Qin, Y.; Wang, Y.-R.; Su, K.-Z.; Han, J.-C.; Mu, Y. Temperature dependence of bioelectrochemical CO2 conversion and methane production with a mixed-culture biocathode. Bioelectrochemistry 2018, 119, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Kuramochi, Y.; Fukushima, N.; Maeda, H.; Sato, K.; Kobayashi, H. Bioelectrochemical Analyses of the Development of a Thermophilic Biocathode Catalyzing Electromethanogenesis. Environ. Sci. Technol. 2015, 49, 1225–1232. [Google Scholar] [CrossRef]
- Van Eerten-Jansen, M.C.A.A.; Veldhoen, A.B.; Plugge, C.M.; Stams, A.J.M.; Buisman, C.J.N.; Ter Heijne, A. Microbial Community Analysis of a Methane-Producing Biocathode in a Bioelectrochemical System. Archaea 2013, 2013, 1–12. [Google Scholar] [CrossRef]
- Van Eerten-Jansen, M.C.A.A.; Heijne, A. Ter; Buisman, C.J.N.; Hamelers, H.V.M. Microbial electrolysis cells for production of methane from CO2: Long-term performance and perspectives. Int. J. Energy Res. 2012, 36, 809–819. [Google Scholar] [CrossRef]
- Muñoz-Aguilar, R.; Molognoni, D.; Bosch-Jimenez, P.; Borràs, E.; Della Pirriera, M.; Luna, Á. Design, Operation, Modeling and Grid Integration of Power-to-Gas Bioelectrochemical Systems. Energies 2018, 11, 1947. [Google Scholar] [CrossRef]
- Wei, J.; Liang, P.; Huang, X. Recent progress in electrodes for microbial fuel cells. Bioresour. Technol. 2011, 102, 9335–9344. [Google Scholar] [CrossRef]
- Appels, L.; Baeyens, J.; Degrève, J.; Dewil, R. Principles and potential of the anaerobic digestion of waste-activated sludge. Prog. Energy Combust. Sci. 2008, 34, 755–781. [Google Scholar] [CrossRef]
- Cui, Z.; DeMontigny, D. A review of CO2 capture using hollow fiber membrane contactors. Carbon Manag. 2013, 4, 69–89. [Google Scholar] [CrossRef]
- Falk-Pedersen, O.; Grønvold, M.S.; Nøkleby, P.; Bjerve, F.; Svendsen, H.F. CO2 Capture with Membrane Contactors. Int. J. Green Energy 2005, 2, 157–165. [Google Scholar] [CrossRef]
- Luis, P.; Van Gerven, T.; Van der Bruggen, B. Recent developments in membrane-based technologies for CO2 capture. Prog. Energy Combust. Sci. 2012, 38, 419–448. [Google Scholar] [CrossRef]
- Zhang, H.-Y.; Wang, R.; Liang, D.T.; Tay, J.H. Modeling and experimental study of CO2 absorption in a hollow fiber membrane contactor. J. Memb. Sci. 2006, 279, 301–310. [Google Scholar] [CrossRef]
- Dindore, V.Y.; Versteeg, G.F. Gas-liquid mass transfer in a cross-flow hollow fiber module: Analytical model and experimental validation. Int. J. Heat Mass Transf. 2005, 48, 3352–3362. [Google Scholar] [CrossRef]
- Al-Marzouqi, M.H.; Marzouk, S.A.M.; El-Naas, M.H.; Abdullatif, N. CO2 Removal from CO2−CH4 Gas Mixture Using Different Solvents and Hollow Fiber Membranes. Ind. Eng. Chem. Res. 2009, 48, 3600–3605. [Google Scholar] [CrossRef]
- Mansourizadeh, A.; Ismail, A.F.; Abdullah, M.S.; Ng, B.C. Preparation of polyvinylidene fluoride hollow fiber membranes for CO2 absorption using phase-inversion promoter additives. J. Memb. Sci. 2010, 355, 200–207. [Google Scholar] [CrossRef]
- Chabanon, E.; Roizard, D.; Favre, E. Membrane contactors for postcombustion carbon dioxide capture: A comparative study of wetting resistance on long time scales. Ind. Eng. Chem. Res. 2011, 50, 8237–8244. [Google Scholar] [CrossRef]
- Sleutels, T.H.J.A.; Hoogland, B.J.; Kuntke, P.; Heijne, A.; Buisman, C.J.N.; Hamelers, H.V.M. Gas-permeable hydrophobic membranes enable transport of CO2 and NH3 to improve performance of bioelectrochemical systems. Environ. Sci. Water Res. Technol. 2016. [Google Scholar] [CrossRef]
- Han, S.-J.; Yoo, M.; Kim, D.-W.; Wee, J.-H. Carbon Dioxide Capture Using Calcium Hydroxide Aqueous Solution as the Absorbent. Energy Fuels 2011, 25, 3825–3834. [Google Scholar] [CrossRef]
- Aymà Maldonado, P.; Galí Mascarilla, D.; Pérez Mejías, L.; Sanchis Pérez, S.; Licon-Bernal, E.; Simón Font, X.; Garcia-Montaño, J. Contactores de membrana para la recuperación de nitrógeno en corrientes líquidas residuales: Efecto de la temperatura y limpieza. Tecnoaqua 2017, 28, 2–10. [Google Scholar]
- Molognoni, D.; Bosch-Jimenez, P.; d D Sirvent, J.; Miles, V.; Della Pirriera, M.; Aliaguilla, M.; Borràs, E. Evaluation of different inoculation strategies for electromethanogenesis reactors. In Proceedings of the 6th Meeting of the International Society for Microbial Electrochemistry and Technology (ISMET 6), Lisbon, Portugal, 3–6 October 2017. [Google Scholar]
- Geppert, F.; Liu, D.; van Eerten-Jansen, M.; Weidner, E.; Buisman, C.; ter Heijne, A. Bioelectrochemical Power-to-Gas: State of the Art and Future Perspectives. Trends Biotechnol. 2016, 34, 879–894. [Google Scholar] [CrossRef]
- Sterner, M. Bioenergy and Renewable Power Methane in Integrated 100% Renewable Energy Systems; Kassel University Press: Kassel, Germany, 2009; ISBN 978-3-89958-798-2. [Google Scholar]
- Del Pilar Anzola Rojas, M.; Mateos, R.; Sotres, A.; Zaiat, M.; Gonzalez, E.R.; Escapa, A.; De Wever, H.; Pant, D. Microbial electrosynthesis (MES) from CO2 is resilient to fluctuations in renewable energy supply. Energy Convers. Manag. 2018, 177, 272–279. [Google Scholar] [CrossRef]








| Electrode | Reaction | Potential (at pH 7) | Notes |
|---|---|---|---|
| AN | 2H2O → 4H+ + O2 + 4e− | +0.82 V vs SHE | Abiotic H2O splitting |
| AN | CH3COO− + 4H2O → 2HCO3− + 9H+ + 8e− | −0.28 V vs SHE | Biotic acetate oxidation |
| CAT | 2H+ + 2e− → H2; CO2 + 4H2 → CH4 + 2H2O | −0.41 V vs SHE | Indirect EMG |
| CAT | HCO3− + 9H+ + 8e− → CH4 + 3H2O | −0.24 V vs SHE | Direct EMG |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Alegre, R.; Ceballos-Escalera, A.; Molognoni, D.; Bosch-Jimenez, P.; Galí, D.; Licon, E.; Della Pirriera, M.; Garcia-Montaño, J.; Borràs, E. Integration of Membrane Contactors and Bioelectrochemical Systems for CO2 Conversion to CH4. Energies 2019, 12, 361. https://doi.org/10.3390/en12030361
Rodríguez-Alegre R, Ceballos-Escalera A, Molognoni D, Bosch-Jimenez P, Galí D, Licon E, Della Pirriera M, Garcia-Montaño J, Borràs E. Integration of Membrane Contactors and Bioelectrochemical Systems for CO2 Conversion to CH4. Energies. 2019; 12(3):361. https://doi.org/10.3390/en12030361
Chicago/Turabian StyleRodríguez-Alegre, Rubén, Alba Ceballos-Escalera, Daniele Molognoni, Pau Bosch-Jimenez, David Galí, Edxon Licon, Monica Della Pirriera, Julia Garcia-Montaño, and Eduard Borràs. 2019. "Integration of Membrane Contactors and Bioelectrochemical Systems for CO2 Conversion to CH4" Energies 12, no. 3: 361. https://doi.org/10.3390/en12030361
APA StyleRodríguez-Alegre, R., Ceballos-Escalera, A., Molognoni, D., Bosch-Jimenez, P., Galí, D., Licon, E., Della Pirriera, M., Garcia-Montaño, J., & Borràs, E. (2019). Integration of Membrane Contactors and Bioelectrochemical Systems for CO2 Conversion to CH4. Energies, 12(3), 361. https://doi.org/10.3390/en12030361