Transparent Luminescent Solar Concentrators Using Ln3+-Based Ionosilicas Towards Photovoltaic Windows
Abstract
:1. Introduction
2. Materials and Methods
3. Results
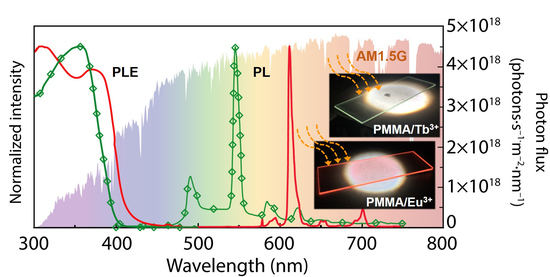
3.1. Optical Characterization
3.2. LSCs
3.3. Modelling
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Huang, X.; Han, S.; Huang, W.; Liu, X. Enhancing solar cell efficiency: the search for luminescent materials as spectral converters. Chem. Soc. Rev. 2013, 42, 173–201. [Google Scholar] [CrossRef] [PubMed]
- McKenna, B.; Evans, R.C. Towards efficient spectral converters through materials design for luminescent solar devices. Adv. Mater. 2017, 29, 1606491. [Google Scholar] [CrossRef] [PubMed]
- Bünzli, J.-C.G.; Chauvin, A.-S. Lanthanides in Solar Energy Conversion. In Handbook on the Physics and Chemistry of Rare-Earths; Bünzli, J.-C.G., Pecharsky, V.K., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 2014; Volume 44, pp. 169–281. [Google Scholar]
- Correia, S.F.; de Zea Bermudez, V.; Ribeiro, S.J.; Andre, P.S.; Ferreira, R.A.; Carlos, L.D. Luminescent solar concentrators: Challenges for lanthanide-based organic-inorganic hybrid materials. J. Mater. Chem. A 2014, 2, 5580–5596. [Google Scholar] [CrossRef]
- Kaniyoor, A.; McKenna, B.; Comby, S.; Evans, R.C. Design and response of high-efficiency, planar, doped luminescent solar concentrators using organic–inorganic di-Ureasil waveguides. Adv. Opt. Mater. 2016, 4, 444–456. [Google Scholar] [CrossRef]
- Weber, W.H.; Lambe, J. Luminescent greenhouse collector for solar-radiation. Appl. Opt. 1976, 15, 2299–2300. [Google Scholar] [CrossRef] [PubMed]
- Reisfeld, R.; Neuman, S. Planar solar energy convertor and concentrator based on uranyl-doped glass. Nature 1978, 274, 144–145. [Google Scholar] [CrossRef]
- Kanellis, M.; de Jong, M.M.; Slooff, L.; Debije, M.G. The solar noise barrier project: 1. Effect of incident light orientation on the performance of a large-scale luminescent solar concentrator noise barrier. Renew. Energy 2017, 103, 647–652. [Google Scholar] [CrossRef] [Green Version]
- Debije, M.G.; Rajkumar, V.A. Direct versus indirect illumination of a prototype luminescent solar concentrator. Sol. Energy 2015, 122, 334–340. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Zhang, Y.; Dong, R.; Luscombe, C.K. Review on the Role of Polymers in Luminescent Solar Concentrators. J. Polym. Sci., Part A: Polym. Chem. 2019, 57, 201–215. [Google Scholar] [CrossRef]
- Debije, M.G.; Tzikas, C.; Rajkumar, V.A.; de Jong, M.M. The solar noise barrier project: 2. The effect of street art on performance of a large scale luminescent solar concentrator prototype. Renew. Energy 2017, 113, 1288–1292. [Google Scholar] [CrossRef]
- Debije, M.G.; Tzikas, C.; de Jong, M.M.; Kanellis, M.; Slooff, L.H. The solar noise barrier project: 3. The effects of seasonal spectral variation, cloud cover and heat distribution on the performance of full-scale luminescent solar concentrator panels. Renew. Energy 2018, 116, 335–343. [Google Scholar] [CrossRef] [Green Version]
- Lim, I.-G.; Kang, S.-W.; Hyoung, C.; Park, K.-H.; Kim, S.-E.; Kang, T.-Y.T.-W.; Park, H.-I.; Hwang, J.-H.; Choi, B.-G.; Kang, T.-Y.T.-W.; et al. Wearable Wireless Power Transmission Apparatus and Wireless Power Transmission Method Using the Same. U.S. Patent US20140015470A1, 16 January 2014. [Google Scholar]
- Frias, A.R.; Pecoraro, E.; Correia, S.F.H.; Minas, L.M.G.; Bastos, A.R.; García-Revilla, S.; Balda, R.; Ribeiro, S.J.L.; André, P.S.; Carlos, L.D.; et al. Sustainable luminescent solar concentrators based on organic–inorganic hybrids modified with chlorophyll. J. Mater. Chem., A 2018, 6, 8712–8723. [Google Scholar] [CrossRef]
- Goetzberger, A.; Greubel, W. Solar-Energy Conversion with Fluorescent Collectors. Appl. Phys. 1977, 14, 123–139. [Google Scholar] [CrossRef]
- Levitt, J.A.; Weber, W.H. Materials for luminescent greenhouse solar collectors. Appl. Opt. 1977, 16, 2684–2689. [Google Scholar] [CrossRef] [PubMed]
- Moraitis, P.; Schropp, R.E.I.; van Sark, W.G.J.H.M. Nanoparticles for Luminescent Solar Concentrators— A review. Opt. Mater. (Amst). 2018, 84, 636–645. [Google Scholar] [CrossRef]
- Brennan, L.J.; Purcell-Milton, F.; McKenna, B.; Watson, T.M.; Gun’ko, Y.K.; Evans, R.C. Large area quantum dot luminescent solar concentrators for use with dye-sensitised solar cells. J. Mater. Chem. A 2018, 6, 2671–2680. [Google Scholar] [CrossRef]
- Shcherbatyuk, G.V.; Inman, R.H.H.; Wang, C.; Winston, R.; Ghosh, S. Viability of using near infrared PbS quantum dots as active materials in luminescent solar concentrators. Appl. Phys. Lett. 2010, 96, 191901. [Google Scholar] [CrossRef]
- Inman, R.H.; Shcherbatyuk, G.V.; Medvedko, D.; Gopinathan, A.; Ghosh, S. Cylindrical luminescent solar concentrators with near-infrared quantum dots. Opt. Express 2011, 19, 24308–24313. [Google Scholar] [CrossRef]
- Zhou, Y.; Benetti, D.; Fan, Z.; Zhao, H.; Ma, D.; Govorov, A.O.; Vomiero, A.; Rosei, F. Near infrared, highly efficient luminescent solar concentrators. Adv. Energy Mater. 2016, 6, 1501913. [Google Scholar] [CrossRef]
- Bottrill, M.; Green, M. Some aspects of quantum dot toxicity. Chem. Commun. 2011, 47, 7039–7050. [Google Scholar] [CrossRef]
- Valizadeh, A.; Mikaeili, H.; Samiei, M.; Farkhani, S.M.; Zarghami, N.; Kouhi, M.; Akbarzadeh, A.; Davaran, S. Quantum dots: synthesis, bioapplications, and toxicity. Nanoscale Res. Lett. 2012, 7, 480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Wang, T.; Qin, X.; Zhang, Z.; Sun, Y.; Liang, H.; Li, H. Large-area flexible, transparent, and highly luminescent films containing lanthanide (III) complex-doped ionic liquids for efficiency enhancement of silicon-based heterojunction solar cell. Prog. Photovoltaics Res. Appl. 2017, 25, 1015–1021. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Wang, T.; Li, Z. Ionic Liquids and Rare Earth Soft Luminescent Materials; Springer: Berlin/Heidelberg, Germany, 2016; pp. 157–178. [Google Scholar]
- Ohno, H. Electrochemical Aspects of Ionic Liquids; Wiley-Interscience: Hoboken, NJ, USA, 2005; ISBN 9780471762515. [Google Scholar]
- Hesemann, P.; Viau, L.; Vioux, A. Silica Ionogels and Ionosilicas. In The Sol-Gel Handbook; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; Volume 2, pp. 487–518. ISBN 9783527334865. [Google Scholar]
- Fan, Z.; Wang, Y.; Xue, Z.; Zhang, L.; Chen, Y.; Zhang, S. Preparation, characterization and luminescence of transparent thin film of ionogels. J. Sol-Gel Sci. Technol. 2014, 72, 328–333. [Google Scholar] [CrossRef]
- Zhou, F.; Wang, T.; Li, Z.; Wang, Y. Transparent and luminescent ionogels composed of Eu3+-coordinated ionic liquids and poly(methyl methacrylate). Luminescence 2015, 30, 1303–1307. [Google Scholar] [CrossRef] [PubMed]
- Meinardi, F.; Colombo, A.; Velizhanin, K.A.; Simonutti, R.; Lorenzon, M.; Beverina, L.; Viswanatha, R.; Klimov, V.I.; Brovelli, S. Large-area luminescent solar concentrators based on “Stokes-shift-engineered” nanocrystals in a mass-polymerized PMMA matrix. Nat. Photonics 2014, 8, 392–399. [Google Scholar] [CrossRef]
- Zettl, M.; Mayer, O.; Klampaftis, E.; Richards, B.S. Investigation of Host Polymers for Luminescent Solar Concentrators. Energy Technol. 2017, 5, 1037–1044. [Google Scholar] [CrossRef] [Green Version]
- Asghar, M.I.; Zhang, J.; Wang, H.; Lund, P.D. Device stability of perovskite solar cells—A review. Renew. Sustain. Energy Rev. 2017, 77, 131–146. [Google Scholar] [CrossRef]
- Djurisic, A.B.; Liu, F.Z.; Tam, H.W.; Wong, M.K.; Ng, A.; Surya, C.; Chen, W.; He, Z.B. Perovskite solar cells—An overview of critical issues. Prog. Quantum Electron. 2017, 53, 1–37. [Google Scholar] [CrossRef]
- Loi, M.A.; Hummelen, J.C. Hybrid solar cells - Perovskites under the Sun. Nat. Mater. 2013, 12, 1087–1089. [Google Scholar] [CrossRef]
- Frias, A.R.; Cardoso, M.A.; Gonçalves, H.; Correia, S.F.H.; Pereira, R.F.P.; Nunes, S.C.; André, P.S.; Carlos, L.D.; de Zea Bermudez, V.; Ferreira, R.A.S. Transparent luminescent down-shifting layers based on Ln3+-based ionosilicas. 2019; Unpublished work. [Google Scholar]
- Swinehart, D.F. The Beer-Lambert Law. J. Chem. Educ. 1962, 39, 333–335. [Google Scholar] [CrossRef]
- Reisfeld, R.; Shamrakov, D.; Jorgensen, C. Photostable solar concentrators based on fluorescent glass-films. Sol. Energy Mater. Sol. Cells 1994, 33, 417–427. [Google Scholar] [CrossRef]
- Correia, S.F.H.; Lima, P.P.; André, P.S.; Ferreira, R.A.S.; Carlos, L.D. High-efficiency luminescent solar concentrators for flexible waveguiding photovoltaics. Sol. Energy Mater. Sol. Cells 2015, 138, 51–57. [Google Scholar] [CrossRef]
- Afifi, H.H.; Sayyah, S.M.; Higazy, H.; El-Kalla, E.H. Study of the emission spectra of poly(methyl methacrylate) films doped with luminescent materials. Acta Polym. 1989, 40, 572–575. [Google Scholar] [CrossRef]
- Lima, P.P.; Nobre, S.S.; Freire, R.O.; Alves Junior, S.; Ferreira, R.A.S.; Pischel, U.; Malta, O.L.; Carlos, L.D. Energy transfer mechanisms in organic-inorganic hybrids incorporating europium(III): A quantitative assessment by light emission spectroscopy. J. Phys. Chem. C 2007, 111, 17627–17634. [Google Scholar] [CrossRef]
- Kai, J.A.; Felinto, M.C.F.C.; Nunes, L.A.O.; Malta, O.L.; Brito, H.F. Intermolecular energy transfer and photostability of luminescence-tuneable multicolour PMMA films doped with lanthanide-beta-diketonate complexes. J. Mater. Chem. 2011, 21, 3796–3802. [Google Scholar] [CrossRef]
- Fernandes, M.; de Zea Bermudez, V.; Ferreira, R.A.S.; Carlos, L.D.; Charas, A.; Morgado, J.; Silva, M.M.; Smith, M.J. Highly Photostable Luminescent Poly ( E-caprolactone ) siloxane Biohybrids Doped with Europium Complexes. Chem. Mater. 2007, 19, 3892–3901. [Google Scholar] [CrossRef]
- Garcia-Torres, J.; Bosch-Jimenez, P.; Torralba-Calleja, E.; Kennedy, M.; Ahmed, H.; Doran, J.; Gutierrez-Tauste, D.; Bautista, L.; Della Pirriera, M. Modulating the photoluminescence of europium-based emitting polymers: Influence of the matrix on the photophysical properties. J. Photochem. Photobiol. A Chem. 2014, 275, 103–113. [Google Scholar] [CrossRef]
- Moudam, O.; Rowan, B.C.; Alamiry, M.; Richardson, P.; Richards, B.S.; Jones, A.C.; Robertson, N. Europium complexes with high total photoluminescence quantum yields in solution and in PMMA. Chem. Commun. 2009, 43, 6649–6651. [Google Scholar] [CrossRef]
- Lunstroot, K.; Driesen, K.; Nockemann, P.; Viau, L.; Mutin, P.H.; Vioux, A.; Binnemans, K. Ionic liquid as plasticizer for europium(III)-doped luminescent poly(methyl methacrylate) films. Phys. Chem. Chem. Phys. 2010, 12, 1879–1885. [Google Scholar] [CrossRef]
- Rondão, R.; Frias, A.R.; Correia, S.F.H.; Fu, L.; de Zea Bermudez, V.; André, P.S.; Ferreira, R.A.S.; Carlos, L.D. High-performance near-infrared luminescent solar concentrators. ACS Appl. Mater. Interfaces 2017, 9, 12540–12546. [Google Scholar] [CrossRef]
- Latva, M.; Takalo, H.; Mukkala, V.-M.; Matachescu, C.; Rodríguez-Ubis, J.C.; Kankare, J. Correlation between the lowest triplet state energy level of the ligand and lanthanide(III) luminescence quantum yield. J. Lumin. 1997, 75, 149–169. [Google Scholar] [CrossRef]
- Shavaleev, N.M.; Scopelliti, R.; Gumy, F.; Bünzli, J.-C.G. Near-Infrared Luminescence of Nine-Coordinate Neodymium Complexes with Benzimidazole-Substituted 8-Hydroxyquinolines. Inorg. Chem. 2008, 47, 9055–9068. [Google Scholar] [CrossRef] [PubMed]
- Biju, S.; Eom, Y.K.; Bünzli, J.-C.G.; Kim, H.K. A new tetrakis β-diketone ligand for NIR emitting LnIII ions: luminescent doped PMMA films and flexible resins for advanced photonic applications. J. Mater. Chem. C 2013, 1, 6935. [Google Scholar] [CrossRef] [Green Version]
- Biju, S.; Gopakumar, N.; Bünzli, J.-C.G.; Scopelliti, R.; Kim, H.K.; Reddy, M.L.P. Brilliant Photoluminescence and Triboluminescence from Ternary Complexes of Dy III and Tb III with 3-Phenyl-4-propanoyl-5-isoxazolonate and a Bidentate Phosphine Oxide Coligand. Inorg. Chem. 2013, 52, 8750–8758. [Google Scholar] [CrossRef] [PubMed]
- Malina, I.; Juhnevics, N.; Kampars, V. Study of thermal and optical properties of dibenzoylmethane Eu(III) organic complexes. Proc. Est. Acad. Sci. 2017, 4017, 493–500. [Google Scholar] [CrossRef]
- Ugale, A.; Kalyani, N.T.; Dhoble, S.J. Reddish orange to blue tunable emission from rare earth β-diketonate Eu(TTA)3dpphen complex for solid state lighting applications. Mater. Sci. Energy Technol. 2019, 2, 57–66. [Google Scholar] [CrossRef]
- Heim, R.; Tsien, R.Y. Engineering green fluorescent protein for improved brightness, longer wavelengths and fluorescence resonance energy transfer. Curr. Biol. 1996, 6, 178–182. [Google Scholar] [CrossRef] [Green Version]
- Butkevich, A.N.; Belov, V.N.; Kolmakov, K.; Sokolov, V.V.; Shojaei, H.; Sidenstein, S.C.; Kamin, D.; Matthias, J.; Vlijm, R.; Engelhardt, J.; et al. Hydroxylated Fluorescent Dyes for Live-Cell Labeling: Synthesis, Spectra and Super-Resolution STED. Chem. Eur. J. 2017, 23, 12114–12119. [Google Scholar] [CrossRef] [Green Version]
- Meinardi, F.; Ehrenberg, S.; Dhamo, L.; Carulli, F.; Mauri, M.; Bruni, F.; Simonutti, R.; Kortshagen, U.; Brovelli, S. Highly efficient luminescent solar concentrators based on earth-abundant indirect-bandgap silicon quantum dots. Nat. Photonics 2017, 11, 177–185. [Google Scholar] [CrossRef]
- Correia, S.F.H.; Lima, P.P.; Pecoraro, E.; Ribeiro, S.J.L.; André, P.S.; Ferreira, R.A.S.; Carlos, L.D. Scale up the collection area of luminescent solar concentrators towards metre-length flexible waveguiding photovoltaics. Prog. Photovoltaics Res. Appl. 2016, 24, 1178–1193. [Google Scholar] [CrossRef]
- Freitas, V.T.; Fu, L.S.; Cojocariu, A.M.; Cattoen, X.; Bartlett, J.R.; Le Parc, R.; Bantignies, J.L.; Man, M.W.C.; Andre, P.S.; Ferreira, R.A.S.; et al. Eu3+-based bridged silsesquioxanes for transparent luminescent solar concentrators. ACS Appl. Mater. Interfaces 2015, 7, 8770–8778. [Google Scholar] [CrossRef] [PubMed]



| Film | τ | O | ε | q | B |
|---|---|---|---|---|---|
| PMMA-Tb | 0.060 ± 0.001 | 9.0 | 2.2 | 0.016 ± 0.002 | 3.5 × 103 |
| PMMA-Eu | 0.553 ± 0.006 | 8.7 | 1.1 | 0.305 ± 0.030 | 3.8 × 104 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frias, A.R.; Cardoso, M.A.; Bastos, A.R.N.; Correia, S.F.H.; André, P.S.; Carlos, L.D.; de Zea Bermudez, V.; Ferreira, R.A.S. Transparent Luminescent Solar Concentrators Using Ln3+-Based Ionosilicas Towards Photovoltaic Windows. Energies 2019, 12, 451. https://doi.org/10.3390/en12030451
Frias AR, Cardoso MA, Bastos ARN, Correia SFH, André PS, Carlos LD, de Zea Bermudez V, Ferreira RAS. Transparent Luminescent Solar Concentrators Using Ln3+-Based Ionosilicas Towards Photovoltaic Windows. Energies. 2019; 12(3):451. https://doi.org/10.3390/en12030451
Chicago/Turabian StyleFrias, Ana R., Marita A. Cardoso, Ana R. N. Bastos, Sandra F. H. Correia, Paulo S. André, Luís D. Carlos, Veronica de Zea Bermudez, and Rute A. S. Ferreira. 2019. "Transparent Luminescent Solar Concentrators Using Ln3+-Based Ionosilicas Towards Photovoltaic Windows" Energies 12, no. 3: 451. https://doi.org/10.3390/en12030451
APA StyleFrias, A. R., Cardoso, M. A., Bastos, A. R. N., Correia, S. F. H., André, P. S., Carlos, L. D., de Zea Bermudez, V., & Ferreira, R. A. S. (2019). Transparent Luminescent Solar Concentrators Using Ln3+-Based Ionosilicas Towards Photovoltaic Windows. Energies, 12(3), 451. https://doi.org/10.3390/en12030451