The Effect of Supercritical CO2 on Shaly Caprocks
Abstract
:1. Introduction
2. Geological Setting
3. Samples and Methods
3.1. Samples
3.2. Exposure to scCO2
3.3. Experimental Approach
4. Results
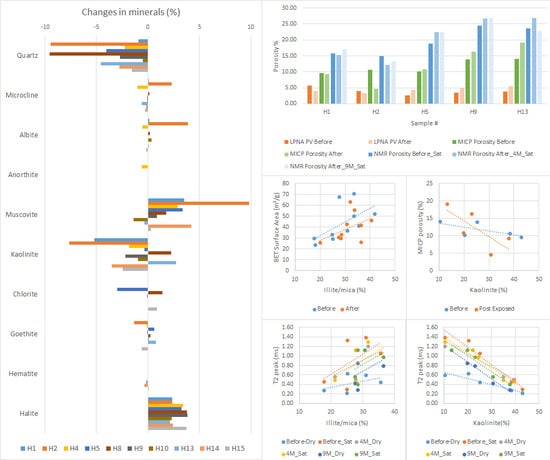
4.1. Mineralogical Changes
4.2. Nuclear Magnetic Resonance
4.3. Low-Pressure Nitrogen Absorption
4.4. Mercury Injection Capillary Pressure
5. Discussion
5.1. The Effect on Mineralogy
5.2. The Effect on Petrophysical Properties
PSD Comparisons
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
List of Acronyms
| CPMG | Carr-Purcell-Meiboom-Gill |
| DFT | Density Functional Theory |
| HPLC | High-performance Liquid Chromatography |
| IPV | Incremental Pore Volume |
| LPNA | Low-pressure Nitrogen Adsorption |
| MICP | Mercury Intrusion Porosimetry |
| NMR | Nuclear Magnetic Resonance |
| PID | Proportional-Integral-Derivative |
| PSD | Pore Size distribution |
| PV | Pore Volume |
| PVT | Pressure-Volume-Temperature |
| SA | Surface Area |
| XRD | X-ray Diffraction |
References
- De Silva, G.P.D.; Ranjith, P.G.; Perera, M.S.A. Geochemical aspects of CO2 sequestration in deep saline aquifers: A review. Fuel 2015, 155, 128–143. [Google Scholar] [CrossRef]
- Kweon, H.; Deo, M. The impact of reactive surface area on brine-rock-carbon dioxide reactions in CO2 sequestration. Fuel 2017, 188, 39–49. [Google Scholar] [CrossRef] [Green Version]
- IPCC. Climate Change 2013, The Physical Science Basis; Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; p. 1535. [Google Scholar]
- Bachu, S.; Gunter, W.; Perkins, E. Aquifer disposal of CO2, Hydrodynamic and mineral trapping. Energy Convers. Manag. 1994, 35, 269–279. [Google Scholar] [CrossRef]
- Gunter, W.D.; Wiwchar, B.; Perkins, E.H. Aquifer disposal of CO2-rich greenhouse gases: Extension of the time scale of experiment for CO2-sequestering reactions by geochemical modelling. Mineral. Petrol. 1997, 59, 121–140. [Google Scholar] [CrossRef]
- Chalbaud, C.; Robin, M.; Lombard, J.M.; Martin, F.; Egermann, P.; Bertin, H. Interfacial tension measurements and wettability evaluation for geological CO2 storage. Adv. Water Resour. 2009, 32, 98–109. [Google Scholar] [CrossRef]
- Busch, A.; Alles, S.; Gensterblum, Y.; Prinz, D.; Dewhurst, D.N.; Raven, M.D.; Stanjek, H.; Krooss, B.M. Carbon dioxide storage potential of shales. Int. J. Greenh. Gas Control 2008, 2, 297–308. [Google Scholar] [CrossRef]
- Wollenweber, J.; Alles, S.; Busch, A.; Krooss, B.M.; Stanjek, H.; Littke, R. Experimental investigation of the CO2 sealing efficiency of caprocks. Int. J. Greenh. Gas Control 2010, 4, 231–241. [Google Scholar] [CrossRef]
- Gaus, I. Role and impact of CO2-rock interactions during CO2 storage in sedimentary rocks. Int. J. Greenh. Gas Control 2010, 4, 73–89. [Google Scholar] [CrossRef]
- Bertier, P.; Swennen, R.; Laenen, B.; Lagrou, D.; Dreesen, R. Experimental identification of CO2–water–rock interactions caused by sequestration of CO2 in Westphalian and Buntsandstein sandstones of the Campine Basin (NE-Belgium). J. Geochem. Explor. 2006, 89, 10–14. [Google Scholar] [CrossRef] [Green Version]
- Kaszuba, J.P.; Janecky, D.R.; Snow, M.G. Carbon dioxide reaction processes in a model brine aquifer at 200 °C and 200 bars: Implications for geologic sequestration of carbon. Appl. Geochem. 2003, 18, 1065–1080. [Google Scholar] [CrossRef]
- Kaszuba, J.P.; Janecky, D.R.; Snow, M.G. Experimental evaluation of mixed fluid reactions between supercritical carbon dioxide and NaCl brine: Relevance to the integrity of a geologic carbon repository. Chem. Geol. 2005, 217, 277–293. [Google Scholar] [CrossRef]
- Kharaka, Y.K.; Cole, D.R.; Thordsen, J.J.; Kakouros, E.; Nance, H.S. Gas–water–rock interactions in sedimentary basins: CO2 sequestration in the Frio Formation, Texas, USA. J. Geochem. Explor. 2006, 89, 183–186. [Google Scholar] [CrossRef]
- Liu, F.; Lu, P.; Griffith, C.; Hedges, S.W.; Soong, Y.; Hellevang, H.; Zhu, C. CO2–brine–caprock interaction: Reactivity experiments on Eau Claire shale and a review of relevant literature. Int. J. Greenh. Gas Control 2012, 7, 153–167. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Kharaka, Y.K.; Thordsen, J.J.; Horita, J.; Karamalidis, A.; Griffith, C.; Hakala, J.A.; Ambats, G.; Cole, D.R.; Phelps, T.J.; et al. CO2–rock–brine interactions in Lower Tuscaloosa Formation at Cranfield CO2 sequestration site, Mississippi, U.S.A. Chem. Geol. 2012, 291, 269–277. [Google Scholar] [CrossRef]
- Rosenbauer, R.J.; Koksalan, T.; Palandri, J.L. Experimental investigation of CO2–brine–rock interactions at elevated temperature and pressure: Implications for CO2 sequestration in deep-saline aquifers. Fuel Process. Technol. 2005, 86, 1581–1597. [Google Scholar] [CrossRef]
- Wigand, M.; Carey, J.W.; Schütt, H.; Spangenberg, E.; Erzinger, J. Geochemical effects of CO2 sequestration in sandstones under simulated in situ conditions of deep saline aquifers. Appl. Geochem. 2008, 23, 2735–2745. [Google Scholar] [CrossRef]
- Credoz, A.; Bildstein, O.; Jullien, M.; Raynal, J.; Pétronin, J.C.; Lillo, M.; Pozo, C.; Geniaut, G. Experimental and modeling study of geochemical reactivity between clayey caprocks and CO2 in geological storage conditions. Energy Procedia 2009, 1, 3445–3452. [Google Scholar] [CrossRef] [Green Version]
- Liu, M. Behaviour of Shale Cap Rock under Exposure to Supercritical CO2. Master’s Thesis, Department of Civil and Environment Engineering, University of Alberta, Edmonton, AB, Canada, 2013; p. 108. [Google Scholar]
- Schlomer, S.; Krooss, B.M. Experimental characterisation of the hydrocarbon sealing efficiency of cap rocks. Mar. Pet. Geol. 1997, 14, 563–578. [Google Scholar] [CrossRef]
- Armitage, P.J.; Faulkner, D.R.; Worden, R.H. Caprock corrosion. Nat. Geosci. 2013, 6, 79–80. [Google Scholar] [CrossRef]
- Yin, H.; Zhou, J.; Jiang, Y.; Xian, X.; Liu, Q. Physical and structural changes in shale associated with supercritical CO2 exposure. Fuel 2016, 184, 289–303. [Google Scholar] [CrossRef]
- Rezaee, R. Shale alteration after exposure to supercritical CO2. Appl. Polym. Sci. 2014, 62, 91–99. [Google Scholar] [CrossRef]
- Szabó, Z.; Hellevang, H.; Király, C.; Sendula, E.; Kónya, P.; Falus, G.; Török, S.; Szabó, C. Experimental-modelling geochemical study of potential CCS caprocks in brine and CO2-saturated brine. Int. J. Greenh. Gas Control 2016, 44, 262–275. [Google Scholar] [CrossRef]
- Alajmi, M.S. Feasibility of Seismic Monitoring Methods for Australian CO2 Storage Projects; Department of Exploration Geophysics, Curtin University: Perth, Australia, 2015; p. 235. [Google Scholar]
- Gibson-Poole, C.M.; Svendsen, L.; Underschultz, J.; Watson, M.N.; Ennis-King, J.; Van Ruth, P.J.; Nelson, E.J.; Daniel, R.F.; Cinar, Y. Site characterisation of a basin-scale CO2 geological storage system: Gippsland Basin, southeast Australia. Environ. Geol. 2007, 54, 1583–1606. [Google Scholar] [CrossRef]
- Delle Piane, C.; Olierook, H.K.H.; Timms, N.E.; Saeedi, A.; Esteban, L.; Rezaee, R.; Mikhaltsevitch, V.; Lebedev, M. Facies-Based Rock Properties Distribution along the Harvey 1 Stratigraphic Well; CSIRO Report Number EP133710; CSIRO: Perth, Australia, 2013. [Google Scholar]
- Olierook, H.K.; Delle Piane, C.; Timms, N.E.; Esteban, L.; Rezaee, R.; Mory, A.J.; Hancock, L. Facies-based rock properties characterization for CO2 sequestration: GSWA Harvey 1 well, Western Australia. Mar. Pet. Geol. 2014, 50, 83–102. [Google Scholar] [CrossRef]
- Soldal, M. Caprock Interaction with CO2 Geomechanical and Geochemical Effects; Department of Geosciences, University of Oslo: Oslo, Norway, 2008. [Google Scholar]
- Manalo, F.; Ding, M.; Bryan, J.; Kantzas, A. Separating the Signals from Clay Bound Water and Heavy Oil in NMR Spectra of Unconsolidated Samples; Society of Petroleum Engineers: Calgary, AB, Canada, 2003. [Google Scholar]
- Matteson, A.; Tomanic, J.P.; Herron, M.M.; Allen, D.F.; Kenyon, W.E. NMR Relaxation of Clay-Brine Mixtures. In Proceedings of the SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 27–30 September 1998. [Google Scholar]
- Bouton, J.C.; Drack, E.D.; Gardner, J.S.; Prammer, M.G. Measurements of Clay-Bound Water and Total Porosity by Magnetic Resonance Logging. In SPE Annual Technical Conference and Exhibition; Society of Petroleum Engineers: Denver, CO, USA, 1996. [Google Scholar]
- Amann, A.; Waschbüsch, M.; Bertier, P.; Busch, A.; Krooss, B.M.; Littke, R. Sealing rock characteristics under the influence of CO2. Energy Procedia 2011, 4, 5170–5177. [Google Scholar] [CrossRef] [Green Version]
- Labani, M.M.; Rezaee, R.; Saeedi, A.; Al Hinai, A. Evaluation of pore size spectrum of gas shale reservoirs using low pressure nitrogen adsorption, gas expansion and mercury porosimetry: A case study from the Perth and Canning Basins, Western Australia. J. Pet. Sci. Eng. 2013, 112, 7–16. [Google Scholar] [CrossRef]
- Rockwater Hydrogelogical and Environmetal Consultants. Harvey 3 Lesueur Formation Fluid Sampling; Southwesthub Geosequestration Project; Department of Mines and Petroleum: Perth, Australia, 2015; p. 24. [Google Scholar]
- Wang, K.; Xu, T.; Wang, F.; Tian, H. Experimental study of CO2–brine–rock interaction during CO2 sequestration in deep coal seams. Int. J. Coal Geol. 2016, 154, 265–274. [Google Scholar] [CrossRef]
- Comisky, J.T.; Santiago, M.; McCollom, B.; Buddhala, A.; Newsham, K.E. Sample Size Effects on the Application of Mercury Injection Capillary Pressure for Determining the Storage Capacity of Tight Gas and Oil Shales; Society of Petroleum Ezngineers: Calgary, AB, Canada, 2011. [Google Scholar]
- Rezaee, R.; Saeedi, A.; Iglauer, S.; Evans, B. Shale alteration after exposure to supercritical CO2. Int. J. Greenh. Gas Control 2017, 62, 91–99. [Google Scholar] [CrossRef]
- Carr, H.Y.; Purcell, E.M. Effects of Diffusion on Free Precession in Nuclear Magnetic Resonance Experiments. Phys. Rev. 1954, 94, 630–638. [Google Scholar] [CrossRef]
- Meiboom, S.; Gill, D. Modified Spin-Echo Method for Measuring Nuclear Relaxation Times. Rev. Sci. Instrum. 1958, 29, 688–691. [Google Scholar] [CrossRef] [Green Version]
- Bustin, R.M.; Clarkson, C.R. Geological controls on coalbed methane reservoir capacity and gas content. Int. J. Coal Geol. 1998, 38, 3–26. [Google Scholar] [CrossRef]
- Rezaee, R.; Saeedi, A.; Iglauer, S.; Evans, B. CarbonNet Dynamic Seal Capacity; Cooperative Research Centre for Greenhouse Gas Technologies Canberra, Curtin University: Perth, Australia, 2013. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Testamanti, M.N. Assessment of Fluid Transport Mechanisms in Shale Gas Reservoirs. Ph.D. Thesis, Curtin University, Perth, Australia, 2018. [Google Scholar]
- Washburn, E.W. Note on a Method of Determining the Distribution of Pore Sizes in a Porous Material. Proc. Natl. Acad. Sci. USA 1921, 7, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izgec, O.; Demiral, B.; Bertin, H.; Akin, S. CO2 injection into saline carbonate aquifer formations I: Laboratory investigation. Transp. Porous Media 2008, 72, 1–24. [Google Scholar] [CrossRef]
- Rathnaweera, T.D.; Ranjith, P.G.; Perera, M.S.; Haque, A. Influence of CO2-Brine Co-injection on CO2 Storage Capacity Enhancement in Deep Saline Aquifers: An Experimental Study on Hawkesbury Sandstone Formation. Energy Fuels 2016, 30, 4229–4243. [Google Scholar] [CrossRef]
- Lahann, R.; Mastalerz, M.; Rupp, J.A.; Drobniak, A. Influence of CO2 on New Albany Shale composition and pore structure. Int. J. Coal Geol. 2013, 108, 2–9. [Google Scholar] [CrossRef]
- Ketzer, J.M.; Iglesias, R.; Einloft, S.; Dullius, J.; Ligabue, R.; De Lima, V. Water-rock-CO2 interactions in saline aquifers aimed for carbon dioxide storage: Experimental and numerical modeling studies of the Rio Bonito Formation (Permian), southern Brazil. Appl. Geochem. 2009, 24, 760–767. [Google Scholar] [CrossRef]
- Rathnaweera, T.D.; Ranjith, P.G.; Perera, M.S.A. Experimental investigation of geochemical and mineralogical effects of CO2 sequestration on flow characteristics of reservoir rock in deep saline aquifers. Sci. Rep. 2016, 6, 19326. [Google Scholar] [CrossRef] [Green Version]
- Alemu, B.L.; Aagaard, P.; Munz, I.A.; Skurtveit, E. Caprock interaction with CO2, A laboratory study of reactivity of shale with supercritical CO2 and brine. Appl. Geochem. 2011, 26, 1975–1989. [Google Scholar] [CrossRef]
- Gunter, W.D.; Perkins, E.H.; Hutcheon, I. Aquifer disposal of acid gases: Modelling of water–rock reactions for trapping of acid wastes. Appl. Geochem. 2000, 15, 1085–1095. [Google Scholar] [CrossRef]
- Moore, J.; Adams, M.; Allis, R.; Lutz, S.; Rauzi, S. Mineralogical and geochemical consequences of the long-term presence of CO2 in natural reservoirs: An example from the Springerville-St. Johns Field, Arizona, and New Mexico, U.S.A. Chem. Geol. 2005, 217, 365–385. [Google Scholar] [CrossRef]
- Gunter, W.D.; Bachu, S.; Benson, S.M. The role of hydrogeological and geochemical trapping in sedimentary basins for secure geological storage of carbon dioxide. Geol. Soc. Spec. Publ. 2004, 233, 129–145. [Google Scholar] [CrossRef]
- Davis, M.C.; Wesolowski, D.J.; Rosenqvist, J.; Brantley, S.L.; Mueller, K.T. Solubility and near-equilibrium dissolution rates of quartz in dilute NaCl solutions at 398-473 K under alkaline conditions. Geochim. Cosmochim. Acta 2011, 75, 401–415. [Google Scholar] [CrossRef]
- Hildenbrand, A.; Urai, J.L. Investigation of the morphology of pore space in mudstones—First results. Mar. Pet. Geol. 2003, 20, 1185–1200. [Google Scholar] [CrossRef]
- Hinai, A.A.; Rezaee, R. Pore Geometry in Gas Shale Reservoirs. In Fundamentals of Gas Shale Reservoirs; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 89–116. [Google Scholar]





















| Samples | Quartz | Microcline | Albite | Anorthite | Illite/Mica | Kaolinite | Chlorite | Goethite | Hematite | Halite | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | Before | After | Before | After | Before | After | Before | After | Before | After | Before | After | |
| H1 | 18.40 | 17.50 | 3.91 | 4.00 | 0.35 | 0.48 | 0.00 | 0.00 | 24.94 | 28.48 | 43.06 | 37.86 | 0.00 | 0.00 | 9.33 | 9.34 | 0.00 | 0.00 | 0.00 | 2.34 |
| H2 | 39.49 | 30.04 | 1.31 | 3.64 | 0.31 | 4.22 | 0.00 | 0.00 | 17.69 | 27.53 | 38.39 | 30.73 | 0.00 | 0.00 | 2.48 | 1.13 | 0.00 | 0.00 | 0.33 | 2.70 |
| H4 | 21.01 | 18.78 | 8.59 | 7.59 | 12.24 | 11.71 | 2.24 | 1.65 | 33.53 | 36.44 | 21.66 | 19.82 | 0.00 | 0.00 | 0.67 | 0.53 | 0.00 | 0.00 | 0.20 | 3.48 |
| H5 | 36.93 | 32.90 | 7.02 | 7.07 | 0.00 | 0.00 | 0.00 | 0.00 | 25.09 | 28.46 | 20.25 | 19.94 | 10.47 | 7.50 | 0.24 | 0.88 | 0.00 | 0.00 | 0.06 | 3.26 |
| H8 | 58.78 | 49.23 | 8.14 | 8.29 | 0.10 | 0.28 | 0.00 | 0.00 | 18.19 | 19.98 | 13.47 | 15.72 | 1.28 | 2.68 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 3.82 |
| H9 | 30.39 | 27.29 | 8.67 | 8.55 | 0.00 | 0.00 | 0.00 | 0.00 | 34.42 | 36.56 | 26.43 | 23.27 | 0.00 | 0.12 | 0.08 | 0.35 | 0.00 | 0.00 | 0.05 | 3.87 |
| H10 | 22.37 | 21.87 | 5.08 | 5.06 | 0.01 | 0.38 | 0.00 | 0.00 | 41.93 | 40.55 | 27.79 | 26.83 | 0.00 | 0.00 | 0.07 | 0.18 | 2.74 | 2.78 | 0.00 | 2.34 |
| H13 | 43.84 | 39.52 | 9.64 | 9.48 | 0.00 | 0.00 | 0.00 | 0.00 | 31.35 | 30.97 | 14.91 | 16.40 | 0.00 | 0.00 | 0.16 | 1.42 | 0.00 | 0.05 | 0.02 | 2.17 |
| H14 | 34.83 | 32.04 | 8.53 | 8.38 | 0.47 | 0.54 | 0.00 | 0.00 | 27.77 | 32.01 | 26.25 | 22.72 | 0.00 | 0.03 | 0.00 | 0.04 | 2.10 | 1.79 | 0.00 | 2.45 |
| H15 | 25.00 | 23.45 | 7.26 | 6.99 | 0.30 | 0.13 | 0.00 | 0.00 | 33.55 | 33.87 | 28.85 | 26.42 | 1.41 | 2.27 | 2.46 | 1.85 | 1.18 | 1.28 | 0.01 | 3.75 |
| Samples | BET Surface Area (m2/g) | Average Pore Width (nm) | DFT Model | |||
|---|---|---|---|---|---|---|
| Micropore Vol. (cm3/100 g) | Mesopore Vol. (cm3/100 g) | Macropore Vol. (cm3/100 g) | Total Pore Vol. (cm3/100 g) | |||
| H1 | 32.96 | 6.868 | 0.347 | 4.859 | 0.453 | 5.659 |
| H2 | 29.71 | 5.467 | 0.347 | 3.500 | 0.214 | 4.060 |
| H4 | 50.29 | 3.718 | 1.226 | 3.186 | 0.263 | 4.675 |
| H5 | 29.29 | 3.521 | 0.332 | 2.186 | 0.061 | 2.578 |
| H8 | 23.88 | 2.889 | 0.405 | 1.299 | 0.021 | 1.725 |
| H9 | 41.34 | 3.406 | 0.731 | 2.720 | 0.070 | 3.520 |
| H10 | 51.96 | 3.157 | 0.987 | 3.067 | 0.047 | 4.101 |
| H13 | 36.54 | 4.210 | 0.620 | 3.033 | 0.193 | 3.846 |
| H14 | 67.61 | 2.904 | 1.654 | 3.205 | 0.049 | 4.908 |
| H15 | 70.63 | 2.844 | 1.495 | 3.504 | 0.023 | 5.022 |
| Samples | BET Surface Area (m2/g) | Average Pore Width (nm) | DFT Model | |||
|---|---|---|---|---|---|---|
| Micropore Vol. (cm3/100 g) | Mesopore Vol. (cm3/100 g) | Macropore Vol. (cm3/100 g) | Total Pore Vol. (cm3/100 g) | |||
| H1 | 33.12 | 4.889 | 0.315 | 3.614 | 0.119 | 4.048 |
| H2 | 30.69 | 4.354 | 0.344 | 2.949 | 0.051 | 3.344 |
| H4 | 26.19 | 4.264 | 0.409 | 2.319 | 0.064 | 2.792 |
| H5 | 29.59 | 5.754 | 0.324 | 3.724 | 0.208 | 4.256 |
| H8 | 25.45 | 3.325 | 0.332 | 1.760 | 0.023 | 2.115 |
| H9 | 41.77 | 4.775 | 0.573 | 4.203 | 0.210 | 4.986 |
| H10 | 46.1 | 4.063 | 0.775 | 3.812 | 0.094 | 4.682 |
| H13 | 42.4 | 5.207 | 0.548 | 4.702 | 0.270 | 5.520 |
| H14 | 63.21 | 3.700 | 1.363 | 4.309 | 0.175 | 5.847 |
| H15 | 55.69 | 4.638 | 1.088 | 5.096 | 0.272 | 6.457 |
| Sample | Porosity (%) | Total Pore Area (m²/g) | Peak Diameter (nm) | Median Pore Diameter (Volume) (nm) | Median Pore Diameter (Area) (nm) | Average Pore Diameter (4V/A) (nm) | Threshold Pressure (psi) | |
|---|---|---|---|---|---|---|---|---|
| H1 | Before | 9.59 | 14.61 | 8.74 | 11.3 | 9.6 | 11.3 | 8349 |
| After | 9.21 | 12.28 | 12.5 | 14.8 | 11.4 | 14.2 | 6708 | |
| H2 | Before | 10.65 | 18.53 | 12.5 | 13.4 | 9.6 | 12.7 | 6310 |
| After | 4.59 | 4.17 | 15.1 | 21.4 | 11.9 | 20.4 | 1736 | |
| H5 | Before | 10.12 | 10.26 | 21.1 | 23.6 | 9.1 | 16.2 | 1897 |
| After | 10.80 | 6.35 | 21.1 | 64.1 | 12.1 | 28.1 | 316 | |
| H9 | Before | 13.93 | 10.89 | 21.1 | 43.6 | 8.9 | 22 | 412 |
| After | 16.32 | 17.2 | 18.1 | 63.5 | 9.4 | 24.1 | 392 | |
| H13 | Before | 14.09 | 13.6 | 21.1 | 30 | 9 | 17.9 | 1179 |
| After | 19.14 | 18.32 | 18.1 | 31.4 | 9.2 | 19.6 | 1090 | |
| Sample. | Depth (m) | P Pore (psi) | T (°C) | ρCO2 (g/cc) | ρbrine (g/cc) | ϒb, CO2 (mN/m) | PThreshold (psi) before | PThreshold (psi) after | CO2 Column Height (m), before | CO2 Column Height (m), after |
|---|---|---|---|---|---|---|---|---|---|---|
| H1 | 1417.5 | 2055 | 64.6 | 0.494 | 0.990 | 28.06 | 8349 | 6708 | 1067.3 | 857.5 |
| H2 | 1412 | 2047 | 64.5 | 0.493 | 0.990 | 28.07 | 6310 | 1736 | 805.4 | 221.6 |
| H5 | 1187.6 | 1722 | 56.9 | 0.443 | 0.993 | 28.68 | 1897 | 316 | 223.3 | 37.2 |
| H9 | 916.3 | 1329 | 47.7 | 0.326 | 0.996 | 30.94 | 412 | 392 | 43.0 | 40.9 |
| H13 | 766.1 | 1111 | 42.6 | 0.229 | 0.998 | 34.01 | 1179 | 1090 | 117.7 | 108.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hadian, P.; Rezaee, R. The Effect of Supercritical CO2 on Shaly Caprocks. Energies 2020, 13, 149. https://doi.org/10.3390/en13010149
Hadian P, Rezaee R. The Effect of Supercritical CO2 on Shaly Caprocks. Energies. 2020; 13(1):149. https://doi.org/10.3390/en13010149
Chicago/Turabian StyleHadian, Pooya, and Reza Rezaee. 2020. "The Effect of Supercritical CO2 on Shaly Caprocks" Energies 13, no. 1: 149. https://doi.org/10.3390/en13010149
APA StyleHadian, P., & Rezaee, R. (2020). The Effect of Supercritical CO2 on Shaly Caprocks. Energies, 13(1), 149. https://doi.org/10.3390/en13010149