A Laboratory Workflow for Characterization of Scaling Deposits in Thermal Wells
Abstract
:1. Introduction
2. Pitzer Theory
3. Materials
4. Methodology
4.1. SEM/EDS Analysis
4.2. ICP-MS Analysis
- The solid sample was crushed using a pestle and mortar to prepare powder.
- To prepare the liquid solution, 0.2 g of the powder was mixed with 8 mL hydrofluoric acid and 2 mL nitric acid in a container.
- The mixture was placed on a hotplate at 130 °C until it became completely dry.
- Five milliliters of hydrochloric acid and 5 mL nitric acid were added to the container. The mixture was heated at 130 °C until it became dry again.
- Ten milliliters of nitric acid (8N) was added to the container, and the mixture was heated at 130 °C for several hours. Acid washing and drying steps with different portions of hydrofluoric and nitric acids were performed to ensure that the scale deposits were completely dissolved in acids.
- To prepare a diluted sample, the mixture and deionized water were mixed in a 15 mL tube.
- One milliliter of the diluted sample was mixed with 0.1 mL nitric acid, 0.1 mL internal standards (In, Bi, and Sc), and 8.8 mL deionized water. These standards were added to ensure that the instrument accurately measured the ions concentration. The final solution was shaken thoroughly to prepare a uniform sample for the ICP-MS analysis.
4.3. Colorimetric Analysis
4.4. Dry Combustion Analysis
5. Results and Discussions
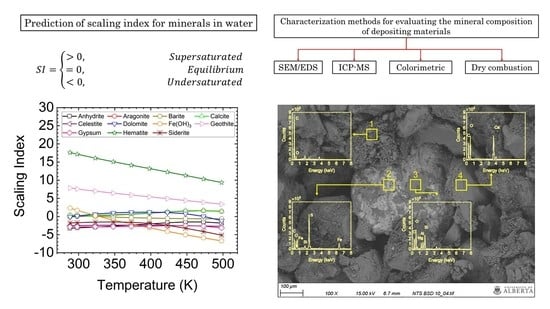
5.1. SI Prediction
5.2. Characterization of Scale Deposits
5.2.1. SEM/EDS Analysis
5.2.2. ICP-MS Analysis
5.2.3. Colorimetric Analysis
5.2.4. Dry Combustion Analysis
6. Conclusions
- The results of the scaling index prediction for minerals showed that Fe-based corrosion products and calcite/aragonite could be the main depositing materials.
- The SEM/EDS analysis visualized the heterogeneity of the scale deposits. The main components were organic matter, Fe-based corrosion products, Mg-based silicates and calcite/aragonite. The ICP-MS analysis agrees with the SEM/EDS visualization results.
- The results of the dry combustion analysis demonstrated the presence of organic matter in the scale deposits. The concentration of organic matter was not negligible; therefore, it is expected that the mixing of the organic matter and inorganic minerals leads to more complex structures of these deposits compared to pure inorganic deposits.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Al-Qasim, A.; Alsubhi, M.; Al-Anazi, A. Heavy Organic Deposit Formation Damage Control, Analysis and Remediation Techniques. In Proceedings of the SPE Kuwait Oil & Gas Show and Conference, Mishref, Kuwait, 13–16 October 2019; Society of Petroleum Engineers: Mishref, Kuwait, 2019. [Google Scholar] [CrossRef]
- Constien, V.G. Evaluation of Formation Damage/Completion Impairment Following Dynamic Filter-Cake Deposition on Unconsolidated Sand. In Proceedings of the SPE International Symposium and Exhibition on Formation Damage Control, Lafayette, LA, USA, 13–15 February 2008; Society of Petroleum Engineers: Lafayette, LA, USA, 2008. [Google Scholar] [CrossRef]
- Moghanloo, R.G.; Davudov, D.; Akita, E. Chapter Six—Formation Damage by Organic Deposition. In Formation Damage During Improved Oil Recovery; Yuan, B., Wood, D.A., Eds.; Gulf Professional Publishing: Oxford, UK, 2018; pp. 243–273. [Google Scholar] [CrossRef]
- Schembre, J.M.; Kovscek, A.R. Mechanism of formation damage at elevated temperature. J. Energy Resour. Technol. Trans. ASME 2005, 127, 171–180. [Google Scholar] [CrossRef]
- Russell, T.; Chequer, L.; Borazjani, S.; You, Z.; Zeinijahromi, A.; Bedrikovetsky, P. Formation Damage by Fines Migration: Mathematical and Laboratory Modeling, Field Cases. In Formation Damage During Improved Oil Recovery; Elsevier: Edinburgh, UK, 2018; pp. 69–175. [Google Scholar] [CrossRef]
- Vetter, O.J.; Kandarpa, V.; Schalge, A.L.; Stratton, M.; Veith, E. Test and Evaluation Methodology for Scale Inhibitor Evaluations. In Proceedings of the SPE International Symposium on Oilfield Chemistry, San Antonio, TX, USA, 4–6 February 1987; Society of Petroleum Engineers: San Antonio, TX, USA, 1987. [Google Scholar]
- Habibi, A.; Fensky, C.; Perri, M.; Roostaei, M.; Fattahpour, V.; Mahmoudi, M.; Ghalambor, A.; Sadrzadeh, M.; Zeng, H. Unplugging Standalone Sand Control Screens with High-power Shock Waves: An Experimental Study. In Proceedings of the SPE International Conference and Exhibition on Formation Damage Control, Lafayette, LA, USA, 19–21 February 2020; Society of Petroleum Engineers: Lafayette, LA, USA, 2020. [Google Scholar] [CrossRef]
- Frenier, W.; Ziauddin, M. Formation, Removal and Inhibition of Inorganic Scale in the Oilfield Environment; SPE: Richardson, TX, USA, 2008; p. 230. [Google Scholar]
- Frenier, W.W.; Hill, D.G. Well Treatment Fluids Comprising Mixed Aldehydes. U.S. Patent 6,399,547, 4 June 2002. [Google Scholar]
- Chilingar, G.V.; Mourhatch, R.; Al-Qahtani, G.D. CHAPTER 6—SCALING. In The Fundamentals of Corrosion and Scaling for Petroleum ’&’ Environmental Engineers; Chilingar, G.V., Mourhatch, R., Al-Qahtani, G.D., Eds.; Gulf Publishing Company: Houston, TX, USA, 2008; pp. 117–139. [Google Scholar] [CrossRef]
- Sarin, P.; Snoeyink, V.L.; Lytle, D.A.; Kriven, W.M. Iron corrosion scales: Model for scale growth, iron release, and colored water formation. J. Environ. Eng. 2004, 130, 364–373. [Google Scholar] [CrossRef]
- Parkhurst, D.L.; Appelo, C.A.J. Description of Input and Examples for PHREEQC Version 3—A Computer Program for Speciation, Batch Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations; U.S. Geological Survey Techniques and Methods: Denver, CO, USA, 2013; p. 497. [Google Scholar]
- Christov, C.; Moller, N. A chemical equilibrium model of solution behavior and solubility in the H-Na-K-Ca-OH-Cl-HSO4-SO4-H2O system to high concentration and temperature. Geochim. Cosmochim. Acta 2004, 68, 3717–3739. [Google Scholar] [CrossRef]
- Pitzer, K.S.; Peiper, J.C.; Busey, R.H. Thermodynamic Properties of Aqueous Sodium Chloride Solutions. J. Phys. Chem. Ref. Data 1984, 13, 1–102. [Google Scholar] [CrossRef] [Green Version]
- Kan, A.T.; Tomson, M.B. Scale prediction for oil and gas production. SPE J. 2012, 17, 362–378. [Google Scholar] [CrossRef]
- Dai, Z.; Kan, A.T.; Shi, W.; Zhang, N.; Zhang, F.; Yan, F.; Bhandari, N.; Zhang, Z.; Liu, Y.; Ruan, G.; et al. Solubility Measurements and Predictions of Gypsum, Anhydrite, and Calcite Over Wide Ranges of Temperature, Pressure, and Ionic Strength with Mixed Electrolytes. Rock Mech. Rock Eng. 2017, 50, 327–339. [Google Scholar] [CrossRef]
- Dai, Z.; Kan, A.; Zhang, F.; Tomson, M. A thermodynamic model for the solubility prediction of barite, calcite, gypsum, and anhydrite, and the association constant estimation of CaSO4(0) ion pair up to 250 °C and 22000 psi. J. Chem. Eng. Data 2015, 60, 766–774. [Google Scholar] [CrossRef]
- Harvie, C.E.; Møller, N.; Weare, J.H. The prediction of mineral solubilities in natural waters: The Na-K-Mg-Ca-H-Cl-SO4-OH-HCO3-CO3-CO2-H2O system to high ionic strengths at 25 °C. Geochim. Cosmochim. Acta 1984, 48, 723–751. [Google Scholar] [CrossRef]
- Appelo, C.A.J. Principles, caveats and improvements in databases for calculating hydrogeochemical reactions in saline waters from 0 to 200 °C and 1 to 1000 atm. Appl. Geochem. 2015, 55, 62–71. [Google Scholar] [CrossRef]
- Pitzer, K.S. Thermodynamics of electrolytes. I. Theoretical basis and general equations. J. Phys. Chem. 1973, 77, 268–277. [Google Scholar] [CrossRef] [Green Version]
- Pitzer, K.S.; Mayorga, G. Thermodynamics of electrolytes. II. Activity and osmotic coefficients for strong electrolytes with one or both ions univalent. J. Phys. Chem. 1973, 77, 2300–2308. [Google Scholar] [CrossRef] [Green Version]
- Pitzer, K.S. Thermodynamics; McGRAW-HILL: New York, NY, USA, 1995. [Google Scholar]
- Dai, Z.; Kan, A.T.; Shi, W.; Yan, F.; Zhang, F.; Bhandari, N.; Ruan, G.; Zhang, Z.; Liu, Y.; Alsaiari, H.A.; et al. Calcite and Barite Solubility Measurements in Mixed Electrolyte Solutions and Development of a Comprehensive Model for Water-Mineral-Gas Equilibrium of the Na-K-Mg-Ca-Ba-Sr-Cl-SO4-CO3-HCO3-CO2(aq)-H2O System up to 250 °C and 1500 bar. Ind. Eng. Chem. Res. 2017, 56, 6548–6561. [Google Scholar] [CrossRef]
- Monnin, C. A thermodynamic model for the solubility of barite and celestite in electrolyte solutions and seawater to 200 °C and to 1 kbar. Chem. Geol. 1999, 153, 187–209. [Google Scholar] [CrossRef]
- Li, J.; Duan, Z. A thermodynamic model for the prediction of phase equilibria and speciation in the H2O-CO2-NaCl-CaCO3-CaSO4 system from 0 to 250 °C, 1 to 1000 bar with NaCl concentrations up to halite saturation. Geochim. Cosmochim. Acta 2011, 75, 4351–4376. [Google Scholar] [CrossRef]
- Shi, W.; Kan, A.T.; Fan, C.; Tomson, M.B. Solubility of barite up to 250 °C and 1500 bar in up to 6 m NaCl solution. Ind. Eng. Chem. Res. 2012, 51, 3119–3128. [Google Scholar] [CrossRef]
- Swinehart, D.F. The Beer-Lambert law. J. Chem. Educ. 1962, 39, 333–335. [Google Scholar] [CrossRef]
- Sauer, M.; Hofkens, J.; Enderlein, J. Basic Principles of Fluorescence Spectroscopy. In Handbook of Fluorescence Spectroscopy and Imaging; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011; Chapter 1; pp. 1–30. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. USEPA Method #325.2; U.S. Environmental Protection Agency: Washington, DC, USA, 1978.
- U.S. Environmental Protection Agency. USEPA Method #375.4; U.S. Environmental Protection Agency: Washington, DC, USA, 1978.
- Official Methods of Analysis of AOAC International. Method 972.43, Micro-Chemical Determination of Carbon, Hydrogen, and Nitrogen, Automated Method. In Official Methods of Analysis of AOAC International, 18th ed.; Revision 1; AOAC International: Gaithersburg, MD, USA, 2006; Chapter 12; pp. 5–6. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Methods of Soil Analysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 961–1010. [Google Scholar]
- Liu, Q.; Whittaker, J.; Marsden, R. Mystery of SAGD Casing Gas Corrosivity and Corrosion Mitigation Strategy. In Proceedings of the NACE International: CORROSION 2015, Dallas, TX, USA, 15–19 March 2015. [Google Scholar]
- Thimm, H. Aquathermolysis and sources of produced gases in SAGD. In Proceedings of the Society of Petroleum Engineers—SPE Heavy Oil Conference Canada, Calgary, AB, Canada, 10–12 June 2014; Volume 1, pp. 641–648. [Google Scholar]
- Pehlke, T. Studies of Aqueous Hydrogen Sulfide Corrosion in Producing SAGD Wells. In Proceedings of the NACE International: CORROSION 2018, Phoenix, AZ, USA, 15–19 April 2018. [Google Scholar]
- Sheikholeslami, R.; Ong, H. Kinetics and thermodynamics of calcium carbonate and calcium sulfate at salinities up to 1.5 M. Desalination 2003, 157, 217–234. [Google Scholar] [CrossRef]
- Fattahpour, V.; Mahmoudi, M.; Roostaei, M.; Cheung, S.; Gong, L.; Qiu, X.; Huang, J.; Velayati, A.; Kyanpour, M.; Alkouh, A.; et al. Evaluation of the Scaling Resistance of Different Coating and Material for Thermal Operations. In Proceedings of the SPE International Heavy Oil Conference and Exhibition, Kuwait City, Kuwait, 10–12 December 2018. [Google Scholar]
- Peramanu, S.; Pruden, B.; Rahimi, P. Molecular weight and specific gravity distributions for Athabasca and Cold Lake bitumens and their saturate, aromatic, resin, and asphaltene fractions. Ind. Eng. Chem. Res. 1999, 38, 3121–3130. [Google Scholar] [CrossRef]
- He, L.; Li, X.; Wu, G.; Lin, F.; Sui, H. Distribution of Saturates, Aromatics, Resins, and Asphaltenes Fractions in the Bituminous Layer of Athabasca Oil Sands. Energy Fuels 2013, 27, 4677–4683. [Google Scholar] [CrossRef]





| Species | A | B | C | D | E |
|---|---|---|---|---|---|
| Na | 266 | 78 | 452 | 380 | 194 |
| K | 15.5 | 16.3 | 19.0 | 20.1 | 3.6 |
| Ca | 9.98 | 1.74 | 3.97 | 4.74 | 56.7 |
| Mg | 3.5 | 0.6 | 1.0 | 1.1 | 21.4 |
| Ba | 0.26 | 0.02 | 0.03 | 0.09 | 0 |
| Sr | 0.4 | 0.04 | 0.17 | 0.23 | 0 |
| Cl | 141 | 26.1 | 323.2 | 233 | 40.4 |
| SO4 | 43 | 33.5 | 313 | 214.4 | 46.1 |
| Fe | 0.07 | 0.28 | 0 | 0 | 0 |
| Alkalinity as HCO3 | 450 | 160 | 400 | 370 | 708 |
| pH | 7.95 | 7.47 | 7.58 | 7.19 | 8.04 |
| Location | ||
|---|---|---|
| Athabasca | 68 | 3660 |
| Cold Lake | 213 | 13,860 |
| Cold Lake | 18 | 15,850 |
| Ion | Concentration, ppm | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Ca | 189,722 | 191,607 | 231,151 | 212,405 | 191,112 |
| Fe | 30,612 | 14,487 | 26,801 | 17,160 | 89,447 |
| Sr | 2485 | 2549 | 2995 | 2781 | 2428 |
| Mg | 5401 | 5358 | 6291 | 5119 | 7763 |
| Ba | 723 | 729 | 799 | 781 | 656 |
| Al | 2851 | 6806 | 3024 | 1491 | 1678 |
| P | 465 | 728 | 863 | 611 | 892 |
| K | 997 | 2933 | 1177 | 438 | 479 |
| Na | 1201 | 2534 | 1422 | 837 | 917 |
| Ti | 183 | 310 | 243 | 126 | 177 |
| Cr | 11 | 9 | 13 | 8 | 17 |
| Mn | 584 | 324 | 529 | 421 | 1211 |
| Ni | 38 | 31 | 45 | 36 | 48 |
| Species | Concentration, ppm | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Cl | 1.44 | 6.63 | 1.47 | 1.87 | 3.14 |
| SO | 4.42 | 8.22 | 3.75 | 10.00 | 16.69 |
| Type of Carbon | Concentration, w/w% | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | Atropine | |
| TC | 15.03 | 14.97 | 14.79 | 16.81 | 15.64 | 70.54 |
| TOC | 9.15 | 9.44 | 8.71 | 11.50 | 9.85 | |
| TIC | 5.88 | 5.54 | 6.08 | 5.31 | 5.79 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habibi, A.; Fensky, C.E.; Roostaei, M.; Mahmoudi, M.; Fattahpour, V.; Zeng, H.; Sadrzadeh, M. A Laboratory Workflow for Characterization of Scaling Deposits in Thermal Wells. Energies 2020, 13, 3184. https://doi.org/10.3390/en13123184
Habibi A, Fensky CE, Roostaei M, Mahmoudi M, Fattahpour V, Zeng H, Sadrzadeh M. A Laboratory Workflow for Characterization of Scaling Deposits in Thermal Wells. Energies. 2020; 13(12):3184. https://doi.org/10.3390/en13123184
Chicago/Turabian StyleHabibi, Ali, Charles E. Fensky, Morteza Roostaei, Mahdi Mahmoudi, Vahidoddin Fattahpour, Hongbo Zeng, and Mohtada Sadrzadeh. 2020. "A Laboratory Workflow for Characterization of Scaling Deposits in Thermal Wells" Energies 13, no. 12: 3184. https://doi.org/10.3390/en13123184
APA StyleHabibi, A., Fensky, C. E., Roostaei, M., Mahmoudi, M., Fattahpour, V., Zeng, H., & Sadrzadeh, M. (2020). A Laboratory Workflow for Characterization of Scaling Deposits in Thermal Wells. Energies, 13(12), 3184. https://doi.org/10.3390/en13123184