Assessing the Alkyl Chain Effect of Ammonium Hydroxides Ionic Liquids on the Kinetics of Pure Methane and Carbon Dioxide Hydrates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Setup and Methods
2.3. Kinetic Measurement Procedure
2.4. Hydrate Kinetic Parameters
2.4.1. Nucleation/Induction Time
2.4.2. Total Gas (CH4 and CO2) Uptake
2.4.3. Initial Hydrate Formation Rate
2.4.4. Relative Inhibition Efficiency (RIE)
3. Results and Discussion
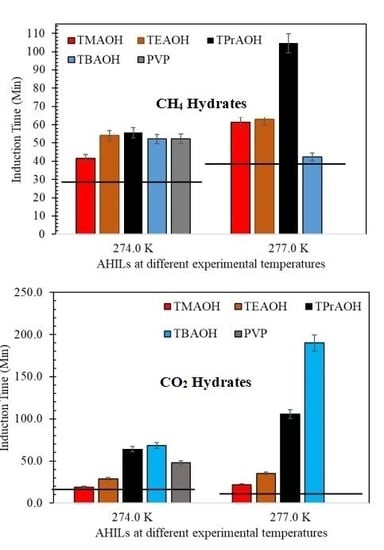
3.1. Effect of AHILs on the Induction Time of CH4 and CO2 Hydrates
3.2. Effect of AHILs on the Initial Formation Rate of CH4 and CO2 Hydrates
3.3. Effect of AHILs on Mole Consumption of CH4 Hydrates
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AILs | Ammonium based Ionic liquids |
| AHILs | Ammonium Hydroxide Ionic liquids |
| [BMIM][BF4] | 1-butyl-3-methyl imidazolium tetrafluoroborate |
| CH4 | Methane |
| CO2 | Carbon Dioxide |
| DFIs | Dual-functional Inhibitors |
| EMIM-CL | 1-ethyl-3-methyl imidazolium chloride |
| [EMPip[[Br] | N-ethyl-N-methyl piperidinium bromide |
| [EMPip][BF4] | N-ethyl-N-methylpiperidinium tetrafluoroborate |
| [EMMor][BF4] | N-ethyl-N-methylmorpholinium tetrafluoroborate |
| IMILs | Imidazolium Ionic Liquids |
| KHIs | kinetic hydrate inhibitors |
| LDHIs | Low dosage hydrate inhibitors |
| TBAOH | Tetrabutylammonium hydroxide |
| THIs | Thermodynamic hydrate inhibitor |
| TPrAOH | Tetrapropylammonium hydroxide |
| TMAOH | Tetramethylammonium hydroxide |
| TMACl | Tetramethyl ammonium chloride |
| TEAOH | Tetraethylammonium hydroxide |
References
- Bavoh, C.B.; Lal, B.; Osei, H.; Sabil, K.M.; Mukhtar, H. A review on the role of amino acids in gas hydrate inhibition, CO2 capture and sequestration, and natural gas storage. J. Nat. Gas Sci. Eng. 2019, 64, 52–71. [Google Scholar] [CrossRef]
- Ripmeester, J.A.; Tse, J.S.; Ratcliffe, C.I.; Powell, B.M. A new clathrate hydrate structure. Nature 1987, 325, 135–136. [Google Scholar] [CrossRef]
- Khan, M.S.; Bavoh, C.B.; Lal, B.; Keong, L.K.; Mellon, N.B.; Bustam, M.A.; Shariff, A.M. Application of electrolyte based model on ionic liquids-methane hydrates phase boundary. IOP Conf. Ser. Mater. Sci. Eng. 2018, 458. [Google Scholar] [CrossRef]
- Taylor, C.E.; Kwan, J.T. Advances in the Study of Gas Hydrates, 1st ed.; Kluwer Academic Publishers: Berlin/Heidelberg, Germany, 2004; ISBN 0-306-48645-8. [Google Scholar]
- Zanota, M.L.; Dicharry, C.; Graciaa, A. Hydrate plug prevention by quaternary ammonium salts. Energy Fuels 2005, 19, 584–590. [Google Scholar] [CrossRef]
- Bavoh, C.B.; Ofei, T.N.; Lal, B.; Sharif, A.M.; Shahpin, M.H.B.A.; Sundramoorthy, J.D. Assessing the impact of an ionic liquid on NaCl/KCl/polymer water-based mud (WBM) for drilling gas hydrate- bearing sediments. J. Mol. Liq. 2019, 100820. [Google Scholar] [CrossRef]
- Bavoh, C.B.; Yuha, Y.B.; Tay, W.H.; Ofei, T.N.; Lal, B.; Mukhtar, H. Experimental and Modelling of the impact of Quaternary Ammonium Salts / Ionic Liquid on the rheological and hydrate inhibition properties of Xanthan gum water-based muds for Drilling Gas Hydrate-Bearing Rocks. J. Pet. Sci. Eng. 2019, 106468. [Google Scholar] [CrossRef]
- Bavoh, C.B.; Ofei, T.N.; Lal, B. Investigating the Potential Cuttings Transport Behavior of Ionic Liquids in Drilling Mud in the Presence of sII Hydrates. Energy Fuels 2020, 34, 2903–2915. [Google Scholar]
- Khan, M.S.; Bavoh, C.; Partoon, B.; Lal, B.; Bustam, M.A.; Shariff, A.M. Thermodynamic effect of ammonium based ionic liquids on CO2 hydrates phase boundary. J. Mol. Liq. 2017, 238, 533–539. [Google Scholar] [CrossRef]
- Bavoh, C.B.; Khan, M.S.; Ting, V.J.; Lal, B.; Ofei, T.N.; Ben-Awuah, J.; Ayoub, M.; Shariff, A.B.M. The Effect of Acidic Gases and Thermodynamic Inhibitors on the Hydrates Phase Boundary of Synthetic Malaysia Natural Gas. IOP Conf. Ser. Mater. Sci. Eng. 2018, 458, 012016. [Google Scholar] [CrossRef]
- Patel, Z.D.; Russum, J. Flow assurance: Chemical inhibition of gas hydrates in deepwater production systems. Multi-Chem 2009, 4, 70–72. [Google Scholar]
- Chun, L.K.; Jaafar, A. Ionic liquid as low dosage hydrate inhibitor for flow assurance in pipeline. Asian J. Sci. Res. 2013, 6, 374–380. [Google Scholar] [CrossRef]
- Nasir, Q.; Lau, K.K.; Lal, B.; Sabil, K.M. Hydrate dissociation condition measurement of CO2—Rich mixed gas in the presence of methanol/ethylene glycol and mixed methanol/ethylene glycol + electrolyte aqueous solution. J. Chem. Eng. Data 2014, 59, 3920–3926. [Google Scholar] [CrossRef]
- Khan, M.S.; Lal, B.; Shariff, A.M.; Mukhtar, H. Ammonium hydroxide ILs as dual-functional gas hydrate inhibitors for binary mixed gas (carbon dioxide and methane) hydrates. J. Mol. Liq. 2019, 274, 33–44. [Google Scholar] [CrossRef]
- Qasim, A.; Khan, M.S.; Lal, B.; Shariff, A.M. Phase equilibrium measurement and modeling approach to quaternary ammonium salts with and without monoethylene glycol for carbon dioxide hydrates. J. Mol. Liq. 2019, 282, 106–114. [Google Scholar] [CrossRef]
- Cha, M.; Shin, K.; Seo, Y.; Shin, J.Y.; Kang, S.P. Catastrophic growth of gas hydrates in the presence of kinetic hydrate inhibitors. J. Phys. Chem. A 2013, 117, 13988–13995. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Cornelius, B.B.; Lal, B.; Bustam, M.A. Kinetic assessment of tetramethyl ammonium hydroxide (ionic liquid) for carbon dioxide, methane and binary mix gas hydrates. In Recent Advances in Ionic Liquids; Rahman, M.M., Ed.; IntechOpen: London, UK, 2018; pp. 159–179. ISBN 978-1-78984-118-3. [Google Scholar]
- Karaaslan, U.; Parlaktuna, M. Kinetic inhibition of methane hydrate by polymers. ACS Div. Fuel Chem. Prepr. 2002, 47, 355–358. [Google Scholar]
- Xiao, C.; Adidharma, H. Dual function inhibitors for methane hydrate. Chem. Eng. Sci. 2009, 64, 1522–1527. [Google Scholar] [CrossRef]
- Peng, X.; Hu, Y.; Liu, Y.; Jin, C.; Lin, H. Separation of ionic liquids from dilute aqueous solutions using the method based on CO2 hydrates. J. Nat. Gas. Chem. 2020, 19, 81–85. [Google Scholar]
- Sabil, K.M.; Nashed, O.; Lal, B.; Ismail, L.; Japper-Jaafar, A. Experimental investigation on the dissociation conditions of methane hydrate in the presence of imidazolium-based ionic liquids. J. Chem. Thermodyn. 2015, 84, 7–13. [Google Scholar] [CrossRef]
- Khan, M.S.; Lal, B.; Bustam, M.A. Gas Hydrate Inhibitors. In Chemical Additives for Gas Hydrates, Green Energy and Technology; Springer: Cham, Switzerland, 2020; ISBN 978-3-030-30749-3. [Google Scholar]
- Tariq, M.; Rooney, D.; Othman, E.; Aparicio, S.; Atilhan, M.; Khraisheh, M. Gas Hydrate Inhibition: A Review of the Role of Ionic Liquids. Ind. Eng. Chem. Res. 2014, 53, 17855–17868. [Google Scholar] [CrossRef]
- Nashed, O.; Sabil, K.M.; Lal, B.; Ismail, L.; Jaafar, A.J. Study of 1-(2-Hydroxyethyle) 3-methylimidazolium halide as thermodynamic inhibitors. Appl. Mech. Mater. 2014, 625, 337–340. [Google Scholar] [CrossRef]
- Avula, V.R.; Gardas, R.L.; Sangwai, J.S. Modeling of methane hydrate inhibition in the presence of green solvent for offshore oil and gas pipeline. Int. Soc. Offshore Polar Eng. 2014, 3, 49–54. [Google Scholar]
- Rufford, T.E.; Smart, S.; Watson, G.C.Y.; Graham, B.F.; Boxall, J.; Diniz da Costa, J.C.; May, E.F. The removal of CO2 and N2 from natural gas: A review of conventional and emerging process technologies. J. Pet. Sci. Eng. 2012, 94, 123–154. [Google Scholar] [CrossRef]
- Nazari, K.; Ahmadi, A.N. A thermodynamic study of methane hydrate formation in the presence of [Bmim][Bf 4] and [Bmim][Ms] ionic liquids. In Proceedings of the 7th Intenational Confrence on Gas Hydrates (ICGH) 2011, Edinbrugh, UK, 17–21 July 2011; p. 9. [Google Scholar]
- Nashed, O.; Dadebayev, D.; Khan, M.S.M.S.; Bavoh, C.B.C.B.; Lal, B.; Shariff, A.M. Experimental and modelling studies on thermodynamic methane hydrate inhibition in the presence of ionic liquids. J. Mol. Liq. 2018, 249, 886–891. [Google Scholar] [CrossRef]
- Bavoh, C.B.; Partoon, B.; Keong, L.K.; Lal, B.; Wilfred, C.D. Eeffect of 1-ethyl-3-methylimidazolium chloride and polyvinylpyrrolidone on kineticss of carbon dioxide hydrates. Int. J. Appl. Chem. 2016, 12, 6–11. [Google Scholar]
- Bavoh, C.B.; Lal, B.; Khan, M.S.; Osei, H.; Ayuob, M. Combined Inhibition Effect of 1-Ethyl-3-methy-limidazolium Chloride + Glycine on Methane Hydrate. J. Phys. Conf. Ser. 2018, 1123, 012060. [Google Scholar] [CrossRef]
- Cha, J.H.; Ha, C.; Kang, S.P.; Kang, J.W.; Kim, K.S. Thermodynamic inhibition of CO2 hydrate in the presence of morpholinium and piperidinium ionic liquids. Fluid Phase Equilib. 2016, 413, 75–79. [Google Scholar] [CrossRef]
- Keshavarz, L.; Javanmardi, J.; Eslamimanesh, A.; Mohammadi, A.H. Experimental measurement and thermodynamic modeling of methane hydrate dissociation conditions in the presence of aqueous solution of ionic liquid. Fluid Phase Equilib. 2013, 354, 312–318. [Google Scholar] [CrossRef]
- Shen, X.; Shi, L.; Long, Z.; Zhou, X.; Liang, D. Experimental study on the kinetic effect of N-butyl-N-methylpyrrolidinium bromide on CO2 hydrate. J. Mol. Liq. 2016, 223, 672–677. [Google Scholar] [CrossRef]
- Kim, K.-S.; Kang, J.W.; Kang, S.-P. Tuning ionic liquids for hydrate inhibition. Chem. Commun. 2011, 47, 6341–6343. [Google Scholar] [CrossRef]
- Khan, M.S.; Lal, B.; Partoon, B.; Kok, L.; Azmi, B. Experimental evaluation of a novel thermodynamic inhibitor for CH4 and CO2 hydrates. Procedia Eng. 2016, 148, 932–940. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.S.; Lal, B.; Bavoh, C.B.; Keong, L.K.; Bustam, A. Influence of ammonium based compounds for gas hydrate mitigation: A short review. Indian J. Sci. Technol. 2017, 10, 1–6. [Google Scholar] [CrossRef]
- Nashed, O.; Koh, J.C.H.; Lal, B. Physical-chemical properties of aqueous TBAOH solution for gas hydrates promotion. Procedia Eng. 2016, 148, 1351–1356. [Google Scholar] [CrossRef] [Green Version]
- Eslamimanesh, A. Thermodynamic Studies on Semi-Clathrate Hydrates of TBAB + Gases Containing Carbon Dioxide. Ph.D. Thesis, Mines ParisTech—Université PSL, Paris, France, 2012. [Google Scholar]
- Shin, B.S.; Kim, E.S.; Kwak, S.K.; Lim, J.S.; Kim, K.S.; Kang, J.W. Thermodynamic inhibition effects of ionic liquids on the formation of condensed carbon dioxide hydrate. Fluid Phase Equilib. 2014, 382, 270–278. [Google Scholar] [CrossRef]
- Khan, M.S.; Partoon, B.; Bavoh, C.; Lal, B.; Mellon, N.B. Influence of tetramethylammonium hydroxide on methane and carbon dioxide gas hydrate phase equilibrium conditions. Fluid Phase Equilib. 2017, 440, 1–8. [Google Scholar] [CrossRef]
- Tariq, M.; Connor, E.; Thompson, J.; Khraisheh, M.; Atilhan, M.; Rooney, D. Doubly dual nature of ammonium-based ionic liquids for methane hydrates probed by rocking-rig assembly. RSC Adv. 2016, 6, 23827–23836. [Google Scholar] [CrossRef]
- Khan, M.S.; Lal, B.; Keong, L.K.; Ahmed, I. Tetramethyl ammonium chloride as dual functional inhibitor for methane and carbon dioxide hydrates. Fuel 2019, 236, 251–263. [Google Scholar] [CrossRef]
- Anantharaj, R.; Banerjee, T. Phase behaviour of 1-ethyl-3-methylimidazolium thiocyanate ionic liquid with catalytic deactivated compounds and water at several temperatures: Experiments and theoretical predictions. Int. J. Chem. Eng. 2011, 2011, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Diedenhofen, M.; Klamt, A. COSMO-RS as a tool for property prediction of IL mixtures—A review. Fluid Phase Equilib. 2010, 294, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Tumba, K.; Reddy, P.; Naidoo, P.; Ramjugernath, D.; Eslamimanesh, A.; Mohammadi, A.H.; Richon, D. Phase equilibria of methane and carbon dioxide clathrate hydrates in the presence of aqueous solutions of tributylmethylphosphonium methylsulfate ionic liquid. J. Chem. Eng. Data 2011, 56, 3620–3629. [Google Scholar] [CrossRef]
- Sami, N.A.; Das, K.; Sangwai, J.S.; Balasubramanian, N. Phase equilibria of methane and carbon dioxide clathrate hydrates in the presence of (methanol + MgCl2) and (ethylene glycol + MgCl2) aqueous solutions. J. Chem. Thermodyn. 2013, 65, 925–928. [Google Scholar] [CrossRef]
- Partoon, B.; Sabil, K.M.; Roslan, H.; Lal, B.; Keong, L.K. Impact of acetone on phase boundary of methane and carbon dioxide mixed hydrates. Fluid Phase Equilib. 2016, 412, 51–56. [Google Scholar] [CrossRef]
- Nashed, O.; Partoon, B.; Lal, B.; Sabil, K.M.; Shariff, A.M. Review the impact of nanoparticles on the thermodynamics and kinetics of gas hydrate formation. J. Nat. Gas. Sci. Eng. 2018, 55, 452–465. [Google Scholar] [CrossRef]
- Bavoh, C.B.; Lal, B.; Ben-Awuah, J.; Khan, M.S.; Ofori-Sarpong, G. Kinetics of mixed amino acid and ionic liquid on CO2 hydrate formation. IOP Conf. Ser. Mater. Sci. Eng. 2019, 495. [Google Scholar] [CrossRef]
- Khan, M.S.; Liew, C.S.; Kurnia, K.A.; Cornelius, B.; Lal, B. Application of COSMO-RS in Investigating Ionic Liquid as Thermodynamic Hydrate Inhibitor for Methane Hydrate. Procedia Eng. 2016, 148, 862–869. [Google Scholar] [CrossRef] [Green Version]
- Sowa, B.; Zhang, X.H.; Hartley, P.G.; Dunstan, D.E.; Kozielski, K.A.; Maeda, N. Formation of ice, tetrahydrofuran hydrate, and methane/propane mixed gas hydrates in strong monovalent salt solutions. Energy Fuels 2014, 11, 6877–6888. [Google Scholar] [CrossRef]
- Zieliński, R.; Ikeda, S.; Nomura, H.; Kato, S. Effect of temperature on micelle formation in aqueous solutions of alkyltrimethylammonium bromides. J. Colloid Interface Sci. 1989, 129, 175–184. [Google Scholar] [CrossRef]
- Alger, D.B. The effect of temperature on the solubility of gases in liquids. J. Chem. Educ. 1992, 69, 62. [Google Scholar] [CrossRef]
- Nashed, O.; Sabil, K.M.; Ismail, L.; Japper-Jaafar, A.; Lal, B. Mean induction time and isothermal kinetic analysis of methane hydrate formation in water and imidazolium based ionic liquid solutions. J. Chem. Thermodyn. 2017, 117, 1–8. [Google Scholar] [CrossRef]
- Koh, C.A.; Westacott, R.E.; Zhang, W.; Hirachand, K.; Creek, J.L.; Soper, A.K. Mechanisms of gas hydrate formation and inhibition. Fluid Phase Equilib. 2002, 194, 143–151. [Google Scholar] [CrossRef]
- Bavoh, C.B.; Lal, B.; Keong, L.K.; Binti Jasamai, M.; Binti Idress, M. Synergic Kinetic Inhibition Effect of EMIM-Cl + PVP on CO2 Hydrate Formation. Procedia Eng. 2016, 148, 1232–1238. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, N.N.; Nguyen, A.V. Hydrophobic effect on gas hydrate formation in the presence of additives. Energy Fuels 2017, 31, 10311–10323. [Google Scholar] [CrossRef]
- Sloan, E.D.; Koh, C.A.; Sloan, E.D. Clathrate Hydrates of Natural Gases, 3rd ed.; CRC Press Taylor & Francis: Boca Raton, FL, USA, 2008; Volume 87, ISBN 9781420008494. [Google Scholar]
- Circone, S.; Stern, L.A.; Kirby, S.H.; Durham, W.B.; Chakoumakos, B.C.; Rawn, C.J.; Rondinone, A.J.; Ishii, Y. CO2 hydrate: Synthesis, composition, structure, dissociation behavior, and a comparison to structure I CH4 hydrate. J. Phys. Chem. B 2003, 107, 5529–5539. [Google Scholar] [CrossRef]
- Sa, J.-H.; Kwak, G.-H.; Han, K.; Ahn, D.; Lee, K.-H. Gas hydrate inhibition by perturbation of liquid water structure. Sci. Rep. 2015, 5, 11526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villano, L.D.; Kommedal, R.; Fijten, M.W.M.; Schubert, U.S.; Hoogenboom, R.; Kelland, M.A. A study of the kinetic hydrate inhibitor performance and seawater biodegradability of a series of poly(2-alkyl-2-oxazoline)s. Energy Fuels 2009, 23, 3665–3673. [Google Scholar] [CrossRef]
- Bhargava, B.L.; Yasaka, Y.; Klein, M.L. Computational studies of room temperature ionic liquid-water mixtures. Chem. Commun. 2011, 47, 6228–6241. [Google Scholar] [CrossRef]
- Oliver, R.C.; Lipfert, J.; Fox, D.A.; Lo, R.H.; Doniach, S.; Columbus, L. Dependence of micelle size and shape on detergent alkyl chain length and head group. PLoS ONE 2013, 8, e62488. [Google Scholar] [CrossRef]
- Kartikawati, N.A.; Safdar, R.; Lal, B.; Mutalib, M.I.B.A.; Shariff, A.M. Measurement and correlation of the physical properties of aqueous solutions of ammonium based ionic liquids. J. Mol. Liq. 2018, 253, 250–258. [Google Scholar] [CrossRef]
- Khan, M.S.; Lal, B.; Keong, L.K.; Sabil, K.M. Experimental evaluation and thermodynamic modelling of AILs alkyl chain elongation on methane riched gas hydrate system. Fluid Phase Equilib. 2018, 473, 300–309. [Google Scholar] [CrossRef]
- Khan, M.S.; Bavoh, C.B.; Partoon, B.; Nashed, O.; Lal, B.; Mellon, N.B. Impacts of ammonium based ionic liquids alkyl chain on thermodynamic hydrate inhibition for carbon dioxide rich binary gas. J. Mol. Liq. 2018, 261, 283–290. [Google Scholar] [CrossRef]








| No. | Chemical | Chemical Formula | Purity |
|---|---|---|---|
| 1 | Water | H2O | Deionized |
| 2 | Methane | CH4 | 99.995 mol% |
| 3 | Carbon Dioxide | CO2 | 99.995 mol% |
| 4 | Tetramethylammonium Hydroxide | TMAOH | 40 wt.% in aqueous solution |
| 5 | Tetraethylammonium Hydroxide | TEAOH | 40 wt.% in aqueous solution |
| 6 | Tetrapropylammonium Hydroxide | TPrAOH | 40 wt.% in aqueous solution |
| 7 | Tetrabutylammonium Hydroxide | TBAOH | 25 wt.% in aqueous solution |
| Gas | Pressure Ranges (MPa) | Temperature (K) |
|---|---|---|
| CH4 | 8.0 | 274.0 and 277.0 |
| CO2 | 3.50 | 274.0 and 277.0 |
| Author | Studied System | RIE | |
|---|---|---|---|
| CH4 | CO2 | ||
| Nashed et al. [54] | [BMIM][CF3SO3] | 0.35 | - |
| Nashed et al. [54] | [BMIM][CH3SO4] | 0.39 | - |
| Nashed et al. [54] | [OH-EMIM] [Br] | 0.45 | - |
| Chun et al. [12] | [EMIM][BF4] | - | 0.27 |
| Bavoh et al. [56] | [EMIM][Cl] | - | 1.06 |
| Khan et al. [42] | TMACl | 0.063 | 2.0 |
| This study | TMAOH | 0.41 | 0.30 |
| This study | TEAOH | 0.85 | 1.00 |
| This study | TPrAOH | 0.89 | 3.50 |
| This study | TBAOH | 0.77 | 3.80 |
| This study | PVP | 0.78 | 1.90 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.S.; Bavoh, C.B.; Rahman, M.A.; Lal, B.; Quainoo, A.K.; Shah Maulud, A. Assessing the Alkyl Chain Effect of Ammonium Hydroxides Ionic Liquids on the Kinetics of Pure Methane and Carbon Dioxide Hydrates. Energies 2020, 13, 3272. https://doi.org/10.3390/en13123272
Khan MS, Bavoh CB, Rahman MA, Lal B, Quainoo AK, Shah Maulud A. Assessing the Alkyl Chain Effect of Ammonium Hydroxides Ionic Liquids on the Kinetics of Pure Methane and Carbon Dioxide Hydrates. Energies. 2020; 13(12):3272. https://doi.org/10.3390/en13123272
Chicago/Turabian StyleKhan, Muhammad Saad, Cornelius Borecho Bavoh, Mohammad Azizur Rahman, Bhajan Lal, Ato Kwamena Quainoo, and Abdulhalim Shah Maulud. 2020. "Assessing the Alkyl Chain Effect of Ammonium Hydroxides Ionic Liquids on the Kinetics of Pure Methane and Carbon Dioxide Hydrates" Energies 13, no. 12: 3272. https://doi.org/10.3390/en13123272
APA StyleKhan, M. S., Bavoh, C. B., Rahman, M. A., Lal, B., Quainoo, A. K., & Shah Maulud, A. (2020). Assessing the Alkyl Chain Effect of Ammonium Hydroxides Ionic Liquids on the Kinetics of Pure Methane and Carbon Dioxide Hydrates. Energies, 13(12), 3272. https://doi.org/10.3390/en13123272