The Role of Bi-Polar Plate Design and the Start-Up Protocol in the Spatiotemporal Dynamics during Solid Oxide Fuel Cell Anode Reduction
Abstract
:1. Introduction
2. Materials and Methods
3. Results
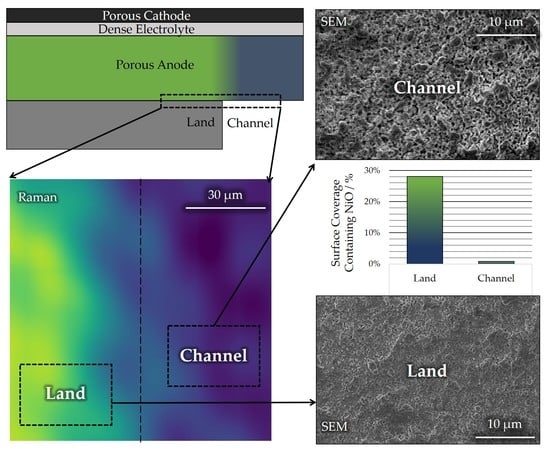
3.1. Chemical Analysis
3.2. Microstructural Analysis
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Choudhury, A.; Chandra, H.; Arora, A. Application of solid oxide fuel cell technology for power generation—A review. Renew. Sust. Energ. Rev. 2013, 20, 430–442. [Google Scholar] [CrossRef]
- Stambouli, A.B.; Traversa, E. Solid oxide fuel cells (SOFCs): A review of an environmentally clean and efficient source of energy. Renew. Sust. Energ. Rev. 2002, 6, 433–455. [Google Scholar] [CrossRef]
- Motoc, A.M.; Valsan, S.; Slobozeanu, A.E.; Corban, M.; Valerini, D.; Prakasam, M.; Botan, M.; Dragut, V.; Vasile, B.S.; Surdu, A.V.; et al. Design, Fabrication, and Characterization of New Materials Based on Zirconia Doped With Mixed Rare Earth Oxides: Review and First Experimental Results. Metals 2020, 10, 746. [Google Scholar] [CrossRef]
- Vecino-Mantilla, S.; Quintero, E.; Fonseca, C.; Gauthier, G.H.; Gauthier-Maradei, P. Catalytic steam reforming of natural gas over a new Ni exsolved Ruddlesden-Popper manganite in SOFC anode conditions. ChemCatChem 2020, 12. [Google Scholar] [CrossRef]
- Gulicovski, J.; Nenadović, S.; Kljajević, L.; Mirković, M.; Nišavić, M.; Kragović, M.; Stojmenović, M. Geopolymer/CeO2 as Solid Electrolyte for IT-SOFC. Polymers 2020, 12, 248. [Google Scholar] [CrossRef] [Green Version]
- Udomsilp, D.; Rechberger, J.; Neubauer, R.; Bischof, C.; Thaler, F.; Schafbauer, W.; Menzler, N.H.; de Haart, L.G.; Nenning, A.; Opitz, A.K.; et al. Metal-Supported Solid Oxide Fuel Cells with Exceptionally High Power Density for Range Extender Systems. Cell Reports Phys. Sci. 2020, 100072. [Google Scholar] [CrossRef]
- Zhu, B.; Fan, L.; Kim, J.S. and Lund, P.D. Electrolyte-Free SOFCs: Materials, Technologies, and Working Principles. In Solid Oxide Fuel Cells: From Electrolyte-Based to Electrolyte-Free Devices; Zhu, B., Raza, R., Fan, L., Sun, C., Eds.; John Wiley & Sons: Weinheim, Germany, 2020; pp. 173–211. [Google Scholar] [CrossRef]
- Hagen, A.; Barfod, R.; Hendriksen, P.V.; Liu, Y.L.; Ramousse, S. Degradation of anode supported SOFCs as a function of temperature and current load. J. Electrochem. Soc. 2006, 153, A1165–A1171. [Google Scholar] [CrossRef]
- Badwal, S.P.S. Stability of solid oxide fuel cell components. Solid State Ion. 2001, 143, 39–46. [Google Scholar] [CrossRef]
- Yokokawa, H.; Tu, H.; Iwanschitz, B.; Mai, A. Fundamental mechanisms limiting solid oxide fuel cell durability. J. Power Sources 2008, 182, 400–412. [Google Scholar] [CrossRef]
- Iwanschitz, B.; Sfeir, J.; Mai, A.; Schütze, M. Degradation of SOFC anodes upon redox cycling: A comparison between Ni/YSZ and Ni/CGO. J. Electrochem. Soc. 2010, 157, B269–B278. [Google Scholar] [CrossRef]
- Robinson, J.B.; Brown, L.D.; Jervis, R.; Taiwo, O.O.; Heenan, T.M.M.; Millichamp, J.; Mason, T.J.; Neville, T.P.; Clague, R.; Eastwood, D.S.; et al. Investigating the effect of thermal gradients on stress in solid oxide fuel cell anodes using combined synchrotron radiation and thermal imaging. J. Power Sources 2015, 288, 473–481. [Google Scholar] [CrossRef]
- Maher, R.C.; Cohen, L.F.; Lohsoontorn, P.; Brett, D.J.L.; Brandon, N.P. Raman spectroscopy as a probe of temperature and oxidation state for gadolinium-doped ceria used in solid oxide fuel cells. J. Phys. Chem. A 2008, 112, 1497–1501. [Google Scholar] [CrossRef]
- Maher, R.C.; Shearing, P.R.; Brightman, E.; Brett, D.J.; Brandon, N.P.; Cohen, L.F. Reduction dynamics of doped ceria, nickel oxide, and cermet composites probed using in situ raman spectroscopy. Adv. Sci. 2016, 3, 1500146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brightman, E.; Maher, R.; Offer, G.J.; Duboviks, V.; Heck, C.; Cohen, L.F.; Brandon, N.P. Designing a miniaturised heated stage for in situ optical measurements of solid oxide fuel cell electrode surfaces, and probing the oxidation of solid oxide fuel cell anodes using in situ Raman spectroscopy. Rev. Sci. Instrum. 2012, 83, 053707. [Google Scholar] [CrossRef]
- Deleebeeck, L.; Shishkin, M.; Addo, P.; Paulson, S.; Molero, H.; Ziegler, T.; Birss, V. Activation of H 2 oxidation at sulphur-exposed Ni surfaces under low temperature SOFC conditions. Phys. Chem. 2014, 16, 9383–9393. [Google Scholar] [CrossRef] [PubMed]
- Brightman, E.; Ivey, D.G.; Brett, D.J.L.; Brandon, N.P. The effect of current density on H2S-poisoning of nickel-based solid oxide fuel cell anodes. J. Power Sources 2011, 196, 7182–7187. [Google Scholar] [CrossRef]
- Nurk, G.; Huthwelker, T.; Braun, A.; Ludwig, C.; Lust, E.; Struis, R.P.W.J. Redox dynamics of sulphur with Ni/GDC anode during SOFC operation at mid-and low-range temperatures: An operando S K-edge XANES study. J. Power Sources 2013, 240, 448–457. [Google Scholar] [CrossRef] [Green Version]
- Izzo, J.R.; Joshi, A.S.; Grew, K.N.; Chiu, W.K.; Tkachuk, A.; Wang, S.H.; Yun, W. Nondestructive reconstruction and analysis of SOFC anodes using X-ray computed tomography at sub-50 nm resolution. J. Electrochem. Soc. 2008, 155, B504–B508. [Google Scholar] [CrossRef] [Green Version]
- Shearing, P.R.; Gelb, J.; Brandon, N.P. X-ray nano computerised tomography of SOFC electrodes using a focused ion beam sample-preparation technique. J. Eur. Ceram. 2010, 30, 1809–1814. [Google Scholar] [CrossRef]
- Guan, Y.; Li, W.; Gong, Y.; Liu, G.; Zhang, X.; Chen, J.; Gelb, J.; Yun, W.; Xiong, Y.; Tian, Y.; et al. Analysis of the three-dimensional microstructure of a solid-oxide fuel cell anode using nano X-ray tomography. J. Power Sources 2011, 196, 1915–1919. [Google Scholar] [CrossRef]
- Laurencin, J.; Quey, R.; Delette, G.; Suhonen, H.; Cloetens, P.; Bleuet, P. Characterisation of Solid Oxide Fuel Cell Ni–8YSZ substrate by synchrotron X-ray nano-tomography: From 3D reconstruction to microstructure quantification. J. Power Sources 2012, 198, 182–189. [Google Scholar] [CrossRef]
- Heenan, T.M.M.; Lu, X.; Iacoviello, F.; Robinson, J.B.; Brett, D.J.L.; Shearing, P.R. Thermally Driven SOFC Degradation in 4D: Part I. Microscale. J. Electrochem. Soc. 2018, 165, F921–F931. [Google Scholar] [CrossRef] [Green Version]
- Heenan, T.M.M.; Lu, X.; Robinson, J.B.; Iacoviello, F.; Brett, D.J.L.; Shearing, P.R. Thermally Driven SOFC Degradation in 4D: Part II. Macroscale. J. Electrochem. Soc. 2018, 165, F932–F941. [Google Scholar] [CrossRef] [Green Version]
- Feser, M.; Gelb, J.; Chang, H.; Cui, H.; Duewer, F.; Lau, S.H.; Tkachuk, A.; Yun, W. Sub-micron resolution CT for failure analysis and process development. Meas. Sci. Tehcnol. 2008, 19, 094001. [Google Scholar] [CrossRef]
- Graham, M.J.; Cohen, M. On the Mechanism of Low-Temperature Oxidation (23°–450° C) of Polycrystalline Nickel. J. Electrochem. Soc. 1972, 119, 879–882. [Google Scholar] [CrossRef]
- Sarantaridis, D.; Atkinson, A. REDOX Cycling of Ni-Based Solid Oxide Fuel Cell Anodes: A Review. Fuel Cells 2007, 7, 246–258. [Google Scholar] [CrossRef]
- Shearing, P.R.; Bradley, R.S.; Gelb, J.; Tariq, F.; Withers, P.J.; Brandon, N.P. Exploring microstructural changes associated with oxidation in Ni–YSZ SOFC electrodes using high resolution X-ray nano-CT computed tomography. Solid State Ion. 2012, 216, 69–72. [Google Scholar] [CrossRef]
- Heenan, T.M.M.; Finegan, D.P.; Tjaden, B.; Lu, X.; Iacoviello, F.; Millichamp, J.; Brett, D.J.; Shearing, P.R. 4D nano-tomography of electrochemical energy devices using lab-based X-ray imaging. Nano Energy 2018, 47, 556–565. [Google Scholar] [CrossRef]
- Ding, K.; Zhu, M.; Han, Z.; Kochetov, V.; Lu, L.; Chen, D. Momentum-species-heat-electrochemistry distribution characteristics within solid oxide fuel cell stack with complex inter-digital fuel channels. Ionics 2020, 1–12. [Google Scholar] [CrossRef]
- Son, J.; Hwang, S.; Hong, S.; Heo, S.; Kim, Y.B. Parameter Study on Solid Oxide Fuel Cell Heat-Up Process to Reaction Starting Temperature. Int. J. Precis. Eng. Manuf.-Green Tech. 2019, 1–11. [Google Scholar] [CrossRef]
- Pan, J.; Yang, J.; Yan, D.; Pu, J.; Chi, B.; Li, J. Effect of thermal cycling on durability of a solid oxide fuel cell stack with external manifold structure. Int. J. Hydrog. Energy 2020, 45, 17927–17934. [Google Scholar] [CrossRef]
- Lyu, Y.; Xie, J.; Wang, D.; Wang, J. Review of cell performance in solid oxide fuel cells. J. Mater. Sci. 2020, 1–24. [Google Scholar] [CrossRef]
- Nabavi, S.A.; Erans, M.; Manović, V. Demonstration of a kW-scale solid oxide fuel cell-calciner for power generation and production of calcined materials. Appl. Energy 2019, 255, 113731. [Google Scholar] [CrossRef]
- Oliphant, T.E. Python for scientific computing. Comput. Sci. Eng. 2007, 9, 10–20. [Google Scholar] [CrossRef] [Green Version]
- Hunter, J.D. Matplotlib: A 2D graphics environment. Comput. Sci. Eng. 2007, 9, 90. [Google Scholar] [CrossRef]
- Lughi, V.; Clarke, D.R. High temperature aging of YSZ coatings and subsequent transformation at low temperature. Surf. Coat. Technol. 2005, 200, 1287–1291. [Google Scholar] [CrossRef]
- Clarke, D.R.; Adar, F. Measurement of the crystallographically transformed zone produced by fracture in ceramics containing tetragonal zirconia. J. Am. Ceram. Soc. 1982, 65, 284–288. [Google Scholar] [CrossRef]
- Mironova-Ulmane, N.; Kuzmin, A.; Steins, I.; Grabis, J.; Sildos, I.; Pärs, M. Raman scattering in nanosized nickel oxide NiO. In Journal of Physics: Conference Series, Proceedings of the Functional Materials and Nanotechnologies, Riga, Latvia, 2–4 April 2007; IOP Publishing: Bristolm, UK, December 2007; Volume 93, No. 1; p. 1012039. [Google Scholar] [CrossRef]
- De Angelis, S.; Jørgensen, P.S.; Tsai, E.H.R.; Holler, M.; Kreka, K.; Bowen, J.R. Three dimensional characterization of nickel coarsening in solid oxide cells via ex-situ ptychographic nano-tomography. J. Power Sources 2018, 383, 72–79. [Google Scholar] [CrossRef] [Green Version]
- Cho, J.I.S.; Marquis, J.; Trogadas, P.; Neville, T.P.; Brett, D.J.L.; Coppens, M.O. Optimizing the architecture of lung-inspired fuel cells. Chem. Eng. Sci. 2020, 215, 115375. [Google Scholar] [CrossRef]
- Grigoriev, S.A.; Millet, P.; Volobuev, S.A.; Fateev, V.N. Optimization of porous current collectors for PEM water electrolysers. Int. J. Hydrog. Energy 2009, 34, 4968–4973. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heenan, T.M.M.; Nabavi, S.A.; Erans, M.; Robinson, J.B.; Kok, M.D.R.; Maier, M.; Brett, D.J.L.; Shearing, P.R.; Manovic, V. The Role of Bi-Polar Plate Design and the Start-Up Protocol in the Spatiotemporal Dynamics during Solid Oxide Fuel Cell Anode Reduction. Energies 2020, 13, 3552. https://doi.org/10.3390/en13143552
Heenan TMM, Nabavi SA, Erans M, Robinson JB, Kok MDR, Maier M, Brett DJL, Shearing PR, Manovic V. The Role of Bi-Polar Plate Design and the Start-Up Protocol in the Spatiotemporal Dynamics during Solid Oxide Fuel Cell Anode Reduction. Energies. 2020; 13(14):3552. https://doi.org/10.3390/en13143552
Chicago/Turabian StyleHeenan, Thomas M. M., Seyed Ali Nabavi, Maria Erans, James B. Robinson, Matthew D. R. Kok, Maximilian Maier, Daniel J. L. Brett, Paul R. Shearing, and Vasilije Manovic. 2020. "The Role of Bi-Polar Plate Design and the Start-Up Protocol in the Spatiotemporal Dynamics during Solid Oxide Fuel Cell Anode Reduction" Energies 13, no. 14: 3552. https://doi.org/10.3390/en13143552
APA StyleHeenan, T. M. M., Nabavi, S. A., Erans, M., Robinson, J. B., Kok, M. D. R., Maier, M., Brett, D. J. L., Shearing, P. R., & Manovic, V. (2020). The Role of Bi-Polar Plate Design and the Start-Up Protocol in the Spatiotemporal Dynamics during Solid Oxide Fuel Cell Anode Reduction. Energies, 13(14), 3552. https://doi.org/10.3390/en13143552