Ferrocene Derivatives Functionalized with Donor/Acceptor (Hetero)Aromatic Substituents: Tuning of Redox Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Information
2.2. Synthesis of Fc Derivatives
3. Results and Discussion
3.1. Design and Synthesis
3.2. Optical Properties of Substituted Ferrocenes
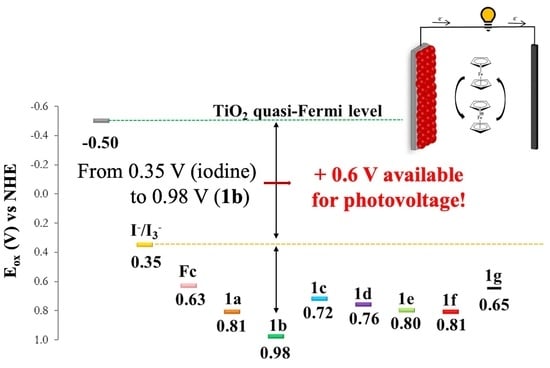
3.3. Electrochemical Properties of Substituted Ferrocenes
3.4. Spectroelectrochemistry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Abbotto, A.; Manfredi, N.; Manfredi, N. Electron-Rich heteroaromatic conjugated polypyridine ruthenium sensitizers for dye-sensitized solar cells. Dalton Trans. 2011, 40, 12421. [Google Scholar] [CrossRef] [PubMed]
- Abbotto, A.; Manfredi, N.; Marinzi, C.; De Angelis, F.; Mosconi, E.; Yum, J.-H.; Xianxi, Z.; Nazeeruddin, M.K.; Grätzel, M. Di-Branched di-anchoring organic dyes for dye-sensitized solar cells. Energy Environ. Sci. 2009, 2, 1094–1101. [Google Scholar] [CrossRef]
- Chen, C.; Liao, J.-Y.; Chi, Z.; Xu, B.; Zhang, X.; Kuang, D.-B.; Zhang, Y.; Liu, S.; Xu, J. Metal-Free organic dyes derived from triphenylethylene for dye-sensitized solar cells: Tuning of the performance by phenothiazine and carbazole. J. Mater. Chem. 2012, 22, 8994–9005. [Google Scholar] [CrossRef]
- Wei, M.; Konishi, Y.; Zhou, H.; Yanagida, M.; Sugihara, H.; Arakawa, H. Highly efficient dye-sensitized solar cells composed of mesoporous titanium dioxide. J. Mater. Chem. 2006, 16, 1287–1293. [Google Scholar] [CrossRef]
- Yella, A.; Lee, H.-W.; Tsao, H.N.; Yi, C.; Chandiran, A.K.; Nazeeruddin, K.; Diau, E.W.-G.; Yeh, C.-Y.; Zakeeruddin, S.M.; Grätzel, M. Porphyrin-Sensitized Solar Cells with Cobalt (II/III)-Based Redox Electrolyte Exceed 12 Percent Efficiency. Science 2011, 334, 629–634. [Google Scholar] [CrossRef]
- Kakiage, K.; Aoyama, Y.; Yano, T.; Oya, K.; Fujisawa, J.-I.; Hanaya, M. Highly-Efficient dye-sensitized solar cells with collaborative sensitization by silyl-anchor and carboxy-anchor dyes. Chem. Commun. 2015, 51, 15894–15897. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, X.; Numata, Y.; Han, L. Highly efficient dye-sensitized solar cells: Progress and future challenges. Energy Environ. Sci. 2013, 6, 1443. [Google Scholar] [CrossRef]
- Nazeeruddin, M.K.; De Angelis, F.; Fantacci, S.; Selloni, A.; Viscardi, G.; Liska, P.; Ito, S.; Takeru, A.B.; Grätzel, M. Combined Experimental and DFT-TDDFT Computational Study of Photoelectrochemical Cell Ruthenium Sensitizers. J. Am. Chem. Soc. 2005, 127, 16835–16847. [Google Scholar] [CrossRef]
- Daeneke, T.; Kwon, T.-H.; Holmes, A.B.; Duffy, N.W.; Bach, U.; Spiccia, L. High-Efficiency dye-sensitized solar cells with ferrocene-based electrolytes. Nat. Chem. 2011, 3, 211–215. [Google Scholar] [CrossRef]
- Daeneke, T.; Mozer, A.J.; Uemura, Y.; Makuta, S.; Fekete, M.; Tachibana, Y.; Koumura, N.; Bach, U.; Spiccia, L. Dye Regeneration Kinetics in Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2012, 134, 16925–16928. [Google Scholar] [CrossRef] [PubMed]
- Sapp, S.A.; Elliott, C.M.; Contado, C.; Caramori, S.; Bignozzi, C.A. Substituted Polypyridine Complexes of Cobalt(II/III) as Efficient Electron-Transfer Mediators in Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2002, 124, 11215–11222. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lan, Z.; Lin, J.; Huang, M.; Huang, Y.; Fan, L.; Luo, G. Electrolytes in Dye-Sensitized Solar Cells. Chem. Rev. 2015, 115, 2136–2173. [Google Scholar] [CrossRef]
- Yanagida, S.; Yu, Y.; Manseki, K. Iodine/Iodide-Free Dye-Sensitized Solar Cells. Acc. Chem. Res. 2009, 42, 1827–1838. [Google Scholar] [CrossRef] [PubMed]
- Cong, J.; Yang, X.; Kloo, L.; Sun, L. Iodine/iodide-free redox shuttles for liquid electrolyte-based dye-sensitized solar cells. Energy Environ. Sci. 2012, 5, 9180. [Google Scholar] [CrossRef]
- Tian, H.; Sun, L. Iodine-Free redox couples for dye-sensitized solar cells. J. Mater. Chem. 2011, 21, 10592. [Google Scholar] [CrossRef]
- Sun, Z.; Liang, M.; Chen, J. Kinetics of Iodine-Free Redox Shuttles in Dye-Sensitized Solar Cells: Interfacial Recombination and Dye Regeneration. Accounts Chem. Res. 2015, 48, 1541–1550. [Google Scholar] [CrossRef]
- Colombo, A.; Di Carlo, G.; Dragonetti, C.; Magni, M.; Biroli, A.O.; Pizzotti, M.; Roberto, D.; Tessore, F.; Benazzi, E.; Bignozzi, C.A.; et al. Coupling of Zinc Porphyrin Dyes and Copper Electrolytes: A Springboard for Novel Sustainable Dye-Sensitized Solar Cells. Inorg. Chem. 2017, 56, 14189–14197. [Google Scholar] [CrossRef] [Green Version]
- Hamann, T.W. The end of iodide? Cobalt complex redox shuttles in DSSCs. Dalton Trans. 2012, 41, 3111. [Google Scholar] [CrossRef]
- Feldt, S.M.; Gibson, E.A.; Gabrielsson, E.; Sun, L.; Boschloo, G.; Hagfeldt, A. Design of Organic Dyes and Cobalt Polypyridine Redox Mediators for High-Efficiency Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2010, 132, 16714–16724. [Google Scholar] [CrossRef]
- Kashif, M.K.; Axelson, J.C.; Duffy, N.W.; Forsyth, C.M.; Chang, C.J.; Long, J.R.; Spiccia, L.; Bach, U. A New Direction in Dye-Sensitized Solar Cells Redox Mediator Development: In Situ Fine-Tuning of the Cobalt(II)/(III) Redox Potential through Lewis Base Interactions. J. Am. Chem. Soc. 2012, 134, 16646–16653. [Google Scholar] [CrossRef] [PubMed]
- Mosconi, E.; Yum, J.-H.; Kessler, F.; García, C.J.G.; Zuccaccia, C.; Cinti, A.; Nazeeruddin, M.K.; Grätzel, M.; De Angelis, F. Cobalt Electrolyte/Dye Interactions in Dye-Sensitized Solar Cells: A Combined Computational and Experimental Study. J. Am. Chem. Soc. 2012, 134, 19438–19453. [Google Scholar] [CrossRef] [PubMed]
- Yum, J.-H.; Baranoff, E.; Kessler, F.; Moehl, T.; Ahmad, S.; Bessho, T.; Marchioro, A.; Ghadiri, E.; Moser, J.-E.; Yi, C.; et al. A cobalt complex redox shuttle for dye-sensitized solar cells with high open-circuit potentials. Nat. Commun. 2012, 3, 631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, K.Y.; Du, C.; Patrick, B.O.; Berlinguette, C.P. High-Voltage Dye-Sensitized Solar Cells Mediated by [Co(2,2′-bipyrimidine)3]z. Inorg. Chem. 2017, 56, 2383–2386. [Google Scholar] [CrossRef]
- Hao, Y.; Liang, M.; Wang, Z.; Wang, L.; Sun, Y.; Sun, Z.; Xue, S. 3,4-ethylenedioxythiophene as an electron donor to construct arylamine sensitizers for highly efficient iodine-free dye-sensitized solar cells. Phys. Chem. Chem. Phys. 2013, 15, 15441. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, H.; Liang, M.; Tan, Y.; Cheng, F.; Sun, Z.; Song, X. Judicious Design of Indoline Chromophores for High-Efficiency Iodine-Free Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2014, 6, 5768–5778. [Google Scholar] [CrossRef]
- Wang, Z.; Liang, M.; Wang, L.; Hao, Y.; Wang, C.; Sun, Z.; Xue, S. New triphenylamine organic dyes containing dithieno[3,2-b:2′,3′-d]pyrrole (DTP) units for iodine-free dye-sensitized solar cells. Chem. Commun. 2013, 49, 5748–5750. [Google Scholar] [CrossRef] [Green Version]
- Tsao, H.N.; Yi, C.; Moehl, T.; Yum, J.-H.; Zakeeruddin, S.M.; Nazeeruddin, M.K.; Grätzel, M. Cyclopentadithiophene Bridged Donor-Acceptor Dyes Achieve High Power Conversion Efficiencies in Dye-Sensitized Solar Cells Based on the tris-Cobalt Bipyridine Redox Couple. ChemSusChem 2011, 4, 591–594. [Google Scholar] [CrossRef]
- Perera, I.R.; Gupta, A.; Xiang, W.; Daeneke, T.; Bach, U.; Evans, R.; Ohlin, C.A.; Spiccia, L. Introducing manganese complexes as redox mediators for dye-sensitized solar cells. Phys. Chem. Chem. Phys. 2014, 16, 12021. [Google Scholar] [CrossRef]
- Daeneke, T.; Mozer, A.J.; Kwon, T.-H.; Duffy, N.W.; Holmes, A.B.; Bach, U.; Spiccia, L. Dye regeneration and charge recombination in dye-sensitized solar cells with ferrocene derivatives as redox mediators. Energy Environ. Sci. 2012, 5, 7090. [Google Scholar] [CrossRef] [Green Version]
- Hamann, T.W.; Farha, O.K.; Hupp, J.T. Outer-Sphere Redox Couples as Shuttles in Dye-Sensitized Solar Cells. Performance Enhancement Based on Photoelectrode Modification via Atomic Layer Deposition. J. Phys. Chem. C 2008, 112, 19756–19764. [Google Scholar] [CrossRef] [Green Version]
- Daeneke, T.; Graf, K.; Watkins, S.; Thelakkat, M.; Bach, U. Infrared Sensitizers in Titania-Based Dye-Sensitized Solar Cells using a Dimethylferrocene Electrolyte. ChemSusChem 2013, 6, 2056–2060. [Google Scholar] [CrossRef] [PubMed]
- Perera, I.R.; Daeneke, T.; Makuta, S.; Yu, Z.; Tachibana, Y.; Mishra, A.; Bäuerle, P.; Ohlin, C.A.; Bach, U.; Spiccia, L. Application of the Tris(acetylacetonato)iron(III)/(II) Redox Couple in p-Type Dye-Sensitized Solar Cells. Angew. Chem. Int. Ed. 2015, 54, 3758–3762. [Google Scholar] [CrossRef] [PubMed]
- Yum, J.-H.; Holcombe, T.; Kim, Y.; Yoon, J.; Rakstys, K.; Nazeeruddin, M.K.; Grätzel, M. Towards high-performance DPP-based sensitizers for DSC applications. Chem. Commun. 2012, 48, 10727–10729. [Google Scholar] [CrossRef] [PubMed]
- Khalid, H.; Wang, L.; Yu, H.; Akram, M.; Abbasi, N.M.; Sun, R.; Saleem, M.; Abdin, Z.U.; Chen, Y. Synthesis of Soluble Ferrocene-Based Polythiophenes and Their Properties. J. Inorg. Organomet. Polym. Mater. 2015, 25, 1511–1520. [Google Scholar] [CrossRef]
- Tajima, K.; Huxur, T.; Imai, Y.; Motoyama, I.; Nakamura, A.; Koshinuma, M. Surface activities of ferrocene surfactants. Colloids Surf. A Physicochem. Eng. Asp. 1995, 94, 243–251. [Google Scholar] [CrossRef]
- Forster, S.; Buckton, G.; Beezer, A.E. The importance of chain length on the wettability and solubility of organic homologs. Int. J. Pharm. 1991, 72, 29–34. [Google Scholar] [CrossRef]
- Wei, X.; Cosimbescu, L.; Xu, W.; Hu, J.Z.; Vijayakumar, M.; Feng, J.; Hu, M.Y.; Deng, X.; Xiao, J.; Liu, J.; et al. Batteries: Towards High-Performance Nonaqueous Redox Flow Electrolyte Via Ionic Modification of Active Species. Adv. Energy Mater. 2015, 5, 1400678. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-Sensitized Solar Cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef]
- Hamann, T.W.; Ondersma, J.W. Dye-Sensitized solar cellredox shuttles. Energy Environ. Sci. 2011, 4, 370–381. [Google Scholar] [CrossRef]
- Miller, T.M.; Ahmed, K.J.; Wrighton, M.S. Complexes of rhenium carbonyl containing ferrocenyl-derived ligands: Tunable electron density at rhenium by control of the redox state of the ferrocenyl ligand. Inorg. Chem. 1989, 28, 2347–2355. [Google Scholar] [CrossRef]
- Braga, D.; D’Addari, D.; Polito, M.; Grepioni, F. Mechanically Induced Expeditious and Selective Preparation of Disubstituted Pyridine/Pyrimidine Ferrocenyl Complexes. Organometallics 2004, 23, 2810–2812. [Google Scholar] [CrossRef]
- Imrie, C.; Loubser, C.; Engelbrecht, P.; McCleland, C.W. The use of a modified Suzuki reaction for the synthesis of monoarylferrocenes. J. Chem. Soc. Perkin Trans. 1 1999, 17, 2513–2523. [Google Scholar] [CrossRef]
- Plyta, Z.F.; Prim, D.; Tranchier, J.-P.; Rose-Munch, F.; Rose, E. Tricarbonyl(η6-arene)chromium and ferrocene complexes linked with aromatic spacers. Tetrahedron Lett. 1999, 40, 6769–6771. [Google Scholar] [CrossRef]
- Shimizu, I.; Umezawa, H.; Kanno, T.; Izumi, T.; Kasahara, A. Synthesis of [0]Orthocyclo[2]orthocyclo[0](1,1′)ferrocenophane and [0]Paracyclo[0](1,1′)ferrocenophane. Bull. Chem. Soc. Jpn. 1983, 56, 2023–2028. [Google Scholar] [CrossRef] [Green Version]
- Nikitin, K.; Müller-Bunz, H.; Ortin, Y.; Muldoon, J.; McGlinchey, M.J. Molecular Dials: Hindered Rotations in Mono- and Diferrocenyl Anthracenes and Triptycenes. J. Am. Chem. Soc. 2010, 132, 17617–17622. [Google Scholar] [CrossRef]
- Li, D.; Zhang, Y.; Jiang, J.; Li, J. Electroactive gold nanoparticles protected by 4-ferrocene thiophenol monolayer. J. Colloid Interface Sci. 2003, 264, 109–113. [Google Scholar] [CrossRef]
- Awtrey, A.D.; Connick, R.E. The Absorption Spectra of I2, I3−, I−, IO3−, S4O6= and S2O3=. Heat of the Reaction I3− = I2 + I−. J. Am. Chem. Soc. 1951, 73, 1842–1843. [Google Scholar] [CrossRef]
- Boschloo, G.; Hagfeldt, A. Characteristics of the Iodide/Triiodide Redox Mediator in Dye-Sensitized Solar Cells. Acc. Chem. Res. 2009, 42, 1819–1826. [Google Scholar] [CrossRef]
- Sarhan, A.A.O.; Ibrahim, M.S.; Kamal, M.M.; Mitobe, K.; Izumi, T. Synthesis, cyclic voltammetry, and UV–Vis studies of ferrocene-dithiafulvalenes as anticipated electron-donor materials. Mon. Chem. Chem. Mon. 2008, 140, 315–323. [Google Scholar] [CrossRef]
- Tauc, J. Optical properties and electronic structure of amorphous Ge and Si. Mater. Res. Bull. 1968, 3, 37–46. [Google Scholar] [CrossRef]
- Pavlishchuk, V.V.; Addison, A.W. Conversion constants for redox potentials measured versus different reference electrodes in acetonitrile solutions at 25 °C. Inorgan. Chim. Acta 2000, 298, 97–102. [Google Scholar] [CrossRef]
- Bockris, J.O.M.; Khan, S.U.M. Surface Electrochemistry—A Molecular Level Approach; Springer: New York, NY, USA, 1993. [Google Scholar]







| Compound | λmax (nm) | ε (M−1 cm−1) |
|---|---|---|
| iodide/triiodide 1 | 353 | 26,400 |
| Fc 2 | 440 | 100 |
| 1a | 452 | 490 ± 5 |
| 1b | 460 | 760 ± 20 |
| 1c | 447 | 380 ± 10 |
| 1d | 484 | 1820 ± 80 |
| 1e | 470 | 1670 ± 100 |
| 1f | 464 | 1960 ± 100 |
| 1g | 447 | 280 ± 10 |
| Eox vs. Fc (V) | Eox vs. NHE 1 (V) | HOMO 2 (eV) | Egapopt (eV) | LUMO (eV) | |
|---|---|---|---|---|---|
| Fc/Fc+ | 0.00 | 0.63 | −5.23 | 2.31 | −2.92 |
| 1a | 0.18 | 0.81 | −5.41 | 2.30 | −3.11 |
| 1b | 0.35 | 0.98 | −5.58 | 2.21 | −3.37 |
| 1c | 0.09 | 0.72 | −5.32 | 2.34 | −2.98 |
| 1d | 0.13 | 0.76 | −5.36 | 2.16 | −3.20 |
| 1e | 0.17 | 0.80 | −5.40 | 2.18 | −3.22 |
| 1f | 0.18 | 0.81 | −5.41 | 2.18 | −3.23 |
| 1g | 0.02 | 0.65 | −5.25 | 2.33 | −2.92 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manfredi, N.; Decavoli, C.; Boldrini, C.L.; Coluccini, C.; Abbotto, A. Ferrocene Derivatives Functionalized with Donor/Acceptor (Hetero)Aromatic Substituents: Tuning of Redox Properties. Energies 2020, 13, 3937. https://doi.org/10.3390/en13153937
Manfredi N, Decavoli C, Boldrini CL, Coluccini C, Abbotto A. Ferrocene Derivatives Functionalized with Donor/Acceptor (Hetero)Aromatic Substituents: Tuning of Redox Properties. Energies. 2020; 13(15):3937. https://doi.org/10.3390/en13153937
Chicago/Turabian StyleManfredi, Norberto, Cristina Decavoli, Chiara L. Boldrini, Carmine Coluccini, and Alessandro Abbotto. 2020. "Ferrocene Derivatives Functionalized with Donor/Acceptor (Hetero)Aromatic Substituents: Tuning of Redox Properties" Energies 13, no. 15: 3937. https://doi.org/10.3390/en13153937
APA StyleManfredi, N., Decavoli, C., Boldrini, C. L., Coluccini, C., & Abbotto, A. (2020). Ferrocene Derivatives Functionalized with Donor/Acceptor (Hetero)Aromatic Substituents: Tuning of Redox Properties. Energies, 13(15), 3937. https://doi.org/10.3390/en13153937