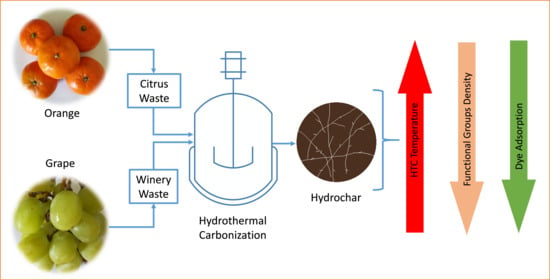
Cationic Dye Adsorption on Hydrochars of Winery and Citrus Juice Industries Residues: Performance, Mechanism, and Thermodynamics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. HTC Experimental Methods
2.3. Characterization of Solid Products
2.4. Adsorption Methods
- qe = equilibrium adsorption capacity in mg/g
- C0 = initial dye concentration in mg/L
- Ce = equilibrium dye concentration in mg/L
- V = volume of the dye solution in mL, and
- W = mass of dry hydrochar used the experiment in g.
- Qmax = maximum adsorption capacity in mg/g
- KL = Langmuir isotherm coefficient
- KF and nF = Freundlich adsorption constants
3. Results and Discussion
3.1. Physico-Chemical Characteristics of Hydrochar
3.2. Effect of Solution’s pH on Adsorption
3.3. Adsorption Isotherms
3.4. Adsorption Thermodynamics
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lazaroiu, G.; Mihaescu, L.; Negreanu, G.; Pana, C.; Pisa, I.; Cernat, A.; Ciupageanu, D.-A. Experimental investigations of innovative biomass energy harnessing solutions. Energies 2018, 11, 3469. [Google Scholar] [CrossRef] [Green Version]
- Ronda, A.; Martín-Lara, M.A.; Calero, M.; Blázquez, G. Complete use of an agricultural waste: Application of untreated and chemically treated olive stone as biosorbent of lead ions and reuse as fuel. Chem. Eng. Res. Des. 2015, 104, 740–751. [Google Scholar] [CrossRef]
- United States Department of Agriculture. Citrus Fruits 2019 Summary; United States Department of Agriculture: Washington, DC, USA, 2019.
- International Organisation of Vine and Wine (OIV). 2019 Statistical Report on World Vitiviniculture; Intergovernmental Organisation: New York, NY, USA, 2019. [Google Scholar]
- Zacharof, M.-P. Grape winery waste as feedstock for bioconversions: Applying the biorefinery concept. Waste Biomass Valorization 2017, 8, 1011–1025. [Google Scholar] [CrossRef] [Green Version]
- Martín, M.A.; Siles, J.A.; Chica, A.F.; Martín, A. Biomethanization of orange peel waste. Bioresour. Technol. 2010, 101, 8993–8999. [Google Scholar] [CrossRef]
- Attard, T.M.; Watterson, B.; Budarin, V.L.; Clark, J.H.; Hunt, A.J. Microwave assisted extraction as an important technology for valorising orange waste. New J. Chem. 2014, 38, 2278–2283. [Google Scholar] [CrossRef]
- Doymaz, İ.; Akgün, N. Study of thin-layer drying of grape wastes. Chem. Eng. Commun. 2009, 196, 890–900. [Google Scholar] [CrossRef]
- Lavelli, V.; Torri, L.; Zeppa, G.; Fiori, L.; Spigno, G. Recovery of winemaking by-products for innovative food application. Ital. J. Food Sci. 2016, 28, 542–564. [Google Scholar]
- Kebaili, M.; Djellali, S.; Radjai, M.; Drouiche, N.; Lounici, H. Valorization of orange industry residues to form a natural coagulant and adsorbent. J. Ind. Eng. Chem. 2018, 64, 292–299. [Google Scholar] [CrossRef]
- Ciuta, S.; Antognoni, S.; Rada, E.C.; Ragazzi, M.; Badea, A.; Cioca, L.I. Respirometric index and biogas potential of different foods and agricultural discarded biomass. Sustainability 2016, 8, 1311. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Heidari, M.; Regmi, B.; Salaudeen, S.; Arku, P.; Thimmannagari, M.; Dutta, A. Hydrothermal carbonization of fruit wastes: A promising technique for generating hydrochar. Energies 2018, 11, 2022. [Google Scholar] [CrossRef] [Green Version]
- Burguete, P.; Corma, A.; Hitzl, M.; Modrego, R.; Ponce, E.; Renz, M. Fuel and chemicals from wet lignocellulosic biomass waste streams by hydrothermal carbonization. Green Chem. 2016, 18, 1051–1060. [Google Scholar] [CrossRef]
- Bandura, A.V.; Lvov, S.N. The ionization constant of water over wide ranges of temperature and density. J. Phys. Chem. Ref. Data 2006, 35, 15–30. [Google Scholar] [CrossRef] [Green Version]
- Reza, M.T.; Rottler, E.; Herklotz, L.; Wirth, B. Hydrothermal carbonization (HTC) of wheat straw: Influence of feedwater pH prepared by acetic acid and potassium hydroxide. Bioresour. Technol. 2015, 182, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Sevilla, M.; Fuertes, A.B. The production of carbon materials by hydrothermal carbonization of cellulose. Carbon 2009, 47, 2281–2289. [Google Scholar] [CrossRef] [Green Version]
- Fu, M.-M.; Mo, C.-H.; Li, H.; Zhang, Y.-N.; Huang, W.-X.; Wong, M.H. Comparison of physicochemical properties of biochars and hydrochars produced from food wastes. J. Clean. Prod. 2019, 236, 117637. [Google Scholar] [CrossRef]
- Saha, N.; Saba, A.; Reza, M.T. Effect of hydrothermal carbonization temperature on pH, dissociation constants, and acidic functional groups on hydrochar from cellulose and wood. J. Anal. Appl. Pyrolysis 2019, 137, 138–145. [Google Scholar] [CrossRef]
- Fang, J.; Gao, B.; Chen, J.; Zimmerman, A.R. Hydrochars derived from plant biomass under various conditions: Characterization and potential applications and impacts. Chem. Eng. J. 2015, 267, 253–259. [Google Scholar] [CrossRef]
- Takaya, C.; Fletcher, L.; Singh, S.; Anyikude, K.; Ross, A. Phosphate and ammonium sorption capacity of biochar and hydrochar from different wastes. Chemosphere 2016, 145, 518–527. [Google Scholar] [CrossRef]
- Román, S.; Nabais, J.M.V.; Laginhas, C.; Ledesma, B.; González, J.F. Hydrothermal carbonization as an effective way of densifying the energy content of biomass. Fuel Process. Technol. 2012, 103, 78–83. [Google Scholar] [CrossRef]
- Zhou, Y.; Engler, N.; Li, Y.; Nelles, M. The influence of hydrothermal operation on the surface properties of kitchen waste-derived hydrochar: Biogas upgrading. J. Clean. Prod. 2020, 259, 121020. [Google Scholar] [CrossRef]
- Basso, D.; Weiss-Hortala, E.; Patuzzi, F.; Baratieri, M.; Fiori, L. In deep analysis on the behavior of grape marc constituents during hydrothermal carbonization. Energies 2018, 11, 1379. [Google Scholar] [CrossRef] [Green Version]
- Saba, A.; McGaughy, K.; Reza, T.M. Techno-Economic Assessment of Co-Hydrothermal Carbonization of a Coal-Miscanthus Blend. Energies 2019, 12, 630. [Google Scholar] [CrossRef] [Green Version]
- Saha, N.; Saba, A.; Saha, P.; McGaughy, K.; Franqui-Villanueva, D.; Orts, W.J.; Hart-Cooper, W.M.; Reza, M.T. Hydrothermal carbonization of various paper mill sludges: An observation of solid fuel properties. Energies 2019, 12, 858. [Google Scholar] [CrossRef] [Green Version]
- Erdogan, E.; Atila, B.; Mumme, J.; Reza, M.T.; Toptas, A.; Elibol, M.; Yanik, J. Characterization of products from hydrothermal carbonization of orange pomace including anaerobic digestibility of process liquor. Bioresour. Technol. 2015, 196, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, G.E.d.; Duarte, M.M.M.B.; Campos, N.F.; Rocha, O.R.S.d.; Silva, V.L.d. Adsorption of azo dyes using peanut hull and orange peel: A comparative study. Environ. Technol. 2014, 35, 1436–1453. [Google Scholar] [CrossRef]
- Fernandez, M.E.; Ledesma, B.; Román, S.; Bonelli, P.R.; Cukierman, A.L. Development and characterization of activated hydrochars from orange peels as potential adsorbents for emerging organic contaminants. Bioresour. Technol. 2015, 183, 221–228. [Google Scholar] [CrossRef]
- Purnomo, C.W.; Castello, D.; Fiori, L. Granular activated carbon from grape seeds hydrothermal char. Appl. Sci. 2018, 8, 331. [Google Scholar] [CrossRef] [Green Version]
- Zúñiga-Muro, N.M.; Bonilla-Petriciolet, A.; Mendoza-Castillo, D.I.; Reynel-Ávila, H.E.; Duran-Valle, C.J.; Ghalla, H.; Sellaoui, L. Recovery of grape waste for the preparation of adsorbents for water treatment: Mercury removal. J. Environ. Chem. Eng. 2020, 8, 103738. [Google Scholar] [CrossRef]
- Singh, A.K.; Ketan, K.; Singh, J.K. Simple and green fabrication of recyclable magnetic highly hydrophobic sorbents derived from waste orange peels for removal of oil and organic solvents from water surface. J. Environ. Chem. Eng. 2017, 5, 5250–5259. [Google Scholar] [CrossRef]
- Ferrentino, R.; Ceccato, R.; Marchetti, V.; Andreottola, G.; Fiori, L. Sewage Sludge Hydrochar: An Option for Removal of Methylene Blue from Wastewater. Appl. Sci. 2020, 10, 3445. [Google Scholar] [CrossRef]
- Fiol, N.; Villaescusa, I. Determination of sorbent point zero charge: Usefulness in sorption studies. Environ. Chem. Lett. 2009, 7, 79–84. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.-J.; Chao, H.-P. Effect of pyrolysis temperatures and times on the adsorption of cadmium onto orange peel derived biochar. Waste Manag. Res. 2016, 34, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Boehm, H.-P. Surface chemical characterization of carbons from adsorption studies. In Adsorption by Carbons; Elsevier: Amsterdam, The Netherlands, 2008; pp. 301–327. [Google Scholar]
- Saha, N.; Xin, D.; Chiu, P.C.; Reza, M.T. Effect of pyrolysis temperature on acidic oxygen-containing functional groups and electron storage capacities of pyrolyzed hydrochars. Acs Sustain. Chem. Eng. 2019, 7, 8387–8396. [Google Scholar] [CrossRef]
- Saha, N.; Reza, M.T. Effect of pyrolysis on basic functional groups of hydrochars. Biomass Convers. Biorefinery 2019, 1–8. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.-J.; Chao, H.-P. Thermodynamic parameters of cadmium adsorption onto orange peel calculated from various methods: A comparison study. J. Environ. Chem. Eng. 2016, 4, 2671–2682. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, X. The unit problem in the thermodynamic calculation of adsorption using the Langmuir equation. Chem. Eng. Commun. 2014, 201, 1459–1467. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.-J.; Chao, H.-P. Fast and efficient adsorption of methylene green 5 on activated carbon prepared from new chemical activation method. J. Environ. Manag. 2017, 188, 322–336. [Google Scholar] [CrossRef]
- Arslan, Y.; Kendüzler, E.; Kabak, B.; Demir, K.; Tomul, F. Determination of Adsorption Characteristics of Orange Peel Activated with Potassium Carbonate for Chromium (III) Removal. J. Turk. Chem. Soc. Sect. A Chem. 2017, 4, 51–64. [Google Scholar]
- Abd El-Latif, M.; Ibrahim, A.M. Adsorption, kinetic and equilibrium studies on removal of basic dye from aqueous solutions using hydrolyzed oak sawdust. Desalin. Water Treat. 2009, 6, 252–268. [Google Scholar] [CrossRef]
- Boumediene, M.; Benaïssa, H.; George, B.; Molina, S.; Merlin, A. Effects of pH and ionic strength on methylene blue removal from synthetic aqueous solutions by sorption onto orange peel and desorption study. J. Mater. Environ. Sci 2018, 9, 1700–1711. [Google Scholar]
- Kuang, Y.; Zhang, X.; Zhou, S. Adsorption of methylene blue in water onto activated carbon by surfactant modification. Water 2020, 12, 587. [Google Scholar] [CrossRef] [Green Version]
- Garg, V.K.; Gupta, R.; Bala Yadav, A.; Kumar, R. Dye removal from aqueous solution by adsorption on treated sawdust. Bioresour. Technol. 2003, 89, 121–124. [Google Scholar] [CrossRef]
- Namasivayam, C.; Prabha, D.; Kumutha, M. Removal of direct red and acid brilliant blue by adsorption on to banana pith. Bioresour. Technol. 1998, 64, 77–79. [Google Scholar] [CrossRef]
- Kermanioryani, M.; Mutalib, M.I.A.; Kurnia, K.A.; Lethesh, K.C.; Krishnan, S.; Leveque, J.-M. Enhancement of π–π aromatic interactions between hydrophobic Ionic Liquids and Methylene Blue for an optimum removal efficiency and assessment of toxicity by microbiological method. J. Clean. Prod. 2016, 137, 1149–1157. [Google Scholar] [CrossRef]
- Sparks, D.L. Environmental Soil Chemistry; Elsevier: Amsterdam, The Netherlands, 2003. [Google Scholar]
- Nasrullah, A.; Saad, B.; Bhat, A.H.; Khan, A.S.; Danish, M.; Isa, M.H.; Naeem, A. Mangosteen peel waste as a sustainable precursor for high surface area mesoporous activated carbon: Characterization and application for methylene blue removal. J. Clean. Prod. 2019, 211, 1190–1200. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.-J.; Chao, H.-P. Insight into adsorption mechanism of cationic dye onto agricultural residues-derived hydrochars: Negligible role of π-π interaction. Korean J. Chem. Eng. 2017, 34, 1708–1720. [Google Scholar] [CrossRef]
- Tan, I.; Hameed, B.; Ahmad, A. Equilibrium and kinetic studies on basic dye adsorption by oil palm fibre activated carbon. Chem. Eng. J. 2007, 127, 111–119. [Google Scholar] [CrossRef]
- Dauenhauer, P.J.; Abdelrahman, O.A. A universal descriptor for the entropy of adsorbed molecules in confined spaces. ACS Cent. Sci. 2018, 4, 1235–1243. [Google Scholar] [CrossRef]





| Sample ID | Solid Yield (%) | Liquid Yield (%) | Gas Yield (%) |
|---|---|---|---|
| CW-H180 | 47.4 | 49.4 | 3.2 |
| CW-H220 | 46.8 | 49.4 | 3.8 |
| CW-H250 | 42.8 | 48.6 | 8.5 |
| WW-H180 | 69.0 | 28.7 | 2.3 |
| WW-H220 | 64.3 | 31.4 | 4.3 |
| WW-H250 | 57.3 | 36.0 | 6.7 |
| Sample ID | pH | pHPZC | BET Surface Area (m2/g) | Density of Surface Functional Groups (µmol/m2) |
|---|---|---|---|---|
| CW-H180 | 4.74 ± 0.06 | 4.72 ± 0.15 | 46.16 ± 0.11 | 23.24 ± 0.22 |
| CW-H220 | 5.24 ± 0.05 | 5.29 ± 0.08 | 47.60 ± 0.90 | 23.36 ± 0.51 |
| CW-H250 | 5.68 ± 0.02 | 5.61 ± 0.04 | 63.82 ± 2.08 | 16.61 ± 0.59 |
| WW-H180 | 4.72 ± 0.06 | 4.22 ± 0.06 | 34.08 ± 1.23 | 32.69 ± 1.39 |
| WW-H220 | 6.52 ± 0.08 | 5.41 ± 0.04 | 41.96 ± 0.88 | 24.33 ± 0.54 |
| WW-H250 | 6.91 ± 0.11 | 5.67 ± 0.06 | 48.04 ± 1.61 | 21.45 ± 0.81 |
| Temperature (°C) | Sample ID | Langmuir Parameters | Freundlich Parameters | ||||
|---|---|---|---|---|---|---|---|
| Qmax (mg/g) | KL | P2 | KF | nF | R2 | ||
| 4 | CW-H180 | 66.23 | 0.23 | 0.99 | 11.16 | 2.56 | 0.87 |
| CW-H220 | 60.24 | 0.21 | 0.99 | 10.15 | 2.79 | 0.86 | |
| CW-H250 | 28.57 | 0.49 | 0.98 | 7.13 | 3.47 | 0.85 | |
| WW-H180 | 36.63 | 0.29 | 0.99 | 7.42 | 3.04 | 0.89 | |
| WW-H220 | 28.57 | 0.40 | 0.99 | 6.93 | 3.52 | 0.87 | |
| WW-H250 | 22.83 | 0.46 | 0.99 | 6.55 | 4.13 | 0.84 | |
| 20 | CW-H180 | 51.02 | 0.34 | 0.99 | 9.72 | 2.68 | 0.92 |
| CW-H220 | 40.00 | 0.46 | 0.99 | 8.96 | 3.13 | 0.91 | |
| CW-H250 | 17.89 | 1.24 | 0.99 | 6.86 | 5.34 | 0.82 | |
| WW-H180 | 36.63 | 1.01 | 0.99 | 11.01 | 3.86 | 0.83 | |
| WW-H220 | 25.97 | 0.64 | 0.99 | 6.99 | 3.85 | 0.85 | |
| WW-H250 | 22.52 | 0.76 | 0.98 | 6.71 | 4.07 | 0.92 | |
| 36 | CW-H180 | 31.65 | 1.54 | 0.98 | 10.25 | 4.00 | 0.93 |
| CW-H220 | 25.64 | 1.10 | 0.99 | 8.30 | 4.33 | 0.92 | |
| CW-H250 | 14.16 | 1.59 | 0.99 | 5.92 | 5.87 | 0.94 | |
| WW-H180 | 28.65 | 1.96 | 0.99 | 9.64 | 4.36 | 0.85 | |
| WW-H220 | 24.63 | 1.16 | 0.98 | 7.82 | 4.20 | 0.89 | |
| WW-H250 | 21.69 | 1.11 | 0.98 | 6.82 | 4.25 | 0.94 | |
| Feedstock | Initial Conc. (ppm) | H180 | H220 | H250 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 4 °C | 20 °C | 36 °C | 4 °C | 20 °C | 36 °C | 4 °C | 20 °C | 36 °C | ||
| CW | 10 | 0.998 | 0.997 | 0.985 | 0.998 | 0.995 | 0.989 | 0.995 | 0.988 | 0.984 |
| 50 | 0.988 | 0.983 | 0.928 | 0.990 | 0.977 | 0.948 | 0.976 | 0.941 | 0.926 | |
| 100 | 0.977 | 0.967 | 0.866 | 0.980 | 0.956 | 0.901 | 0.953 | 0.889 | 0.863 | |
| 200 | 0.955 | 0.936 | 0.764 | 0.960 | 0.915 | 0.820 | 0.911 | 0.800 | 0.758 | |
| 300 | 0.934 | 0.907 | 0.684 | 0.941 | 0.878 | 0.752 | 0.872 | 0.728 | 0.677 | |
| 400 | 0.914 | 0.880 | 0.619 | 0.923 | 0.844 | 0.695 | 0.836 | 0.667 | 0.611 | |
| 500 | 0.895 | 0.854 | 0.565 | 0.906 | 0.812 | 0.645 | 0.803 | 0.616 | 0.557 | |
| WW | 10 | 0.997 | 0.990 | 0.981 | 0.996 | 0.994 | 0.989 | 0.995 | 0.992 | 0.989 |
| 50 | 0.986 | 0.952 | 0.911 | 0.981 | 0.969 | 0.945 | 0.978 | 0.964 | 0.947 | |
| 100 | 0.972 | 0.908 | 0.836 | 0.962 | 0.940 | 0.896 | 0.956 | 0.930 | 0.900 | |
| 200 | 0.946 | 0.831 | 0.718 | 0.927 | 0.887 | 0.811 | 0.916 | 0.869 | 0.818 | |
| 300 | 0.920 | 0.767 | 0.630 | 0.894 | 0.839 | 0.741 | 0.879 | 0.815 | 0.750 | |
| 400 | 0.897 | 0.711 | 0.560 | 0.863 | 0.797 | 0.682 | 0.845 | 0.768 | 0.692 | |
| 500 | 0.874 | 0.663 | 0.505 | 0.835 | 0.758 | 0.632 | 0.813 | 0.726 | 0.643 | |
| Sample ID | Temperature (°C) | Van’t Hoff Equation | KC | ΔG (KJ/mol) | ΔH (KJ/mol) | ΔS (J/mol) |
|---|---|---|---|---|---|---|
| CW-H180 | 4 | y = −4977.2x + 33.0 R2 = 0.873 | 4162272 | −34.66 | 41.38 | 274.51 |
| 20 | 6072126 | −39.05 | ||||
| 36 | 27363558 | −43.44 | ||||
| CW-H220 | 4 | y = −4452.3x + 31.2 R2 = 0.997 | 3681327 | −34.77 | 37.02 | 259.14 |
| 20 | 8233615 | −38.91 | ||||
| 36 | 19501840 | −43.06 | ||||
| CW-H250 | 4 | y = −3187.8x + 27.6 R2 = 0.916 | 8689631 | −37.02 | 26.50 | 229.33 |
| 20 | 22149969 | −40.69 | ||||
| 36 | 28290480 | −44.36 | ||||
| WW-H180 | 4 | y = −5154.8x + 34.1 R2 = 0.978 | 5112033 | −35.75 | 42.90 | 283.93 |
| 20 | 18015640 | −40.29 | ||||
| 36 | 34805250 | −44.84 | ||||
| WW-H220 | 4 | y = −2877.2x + 26.1 R2 = 0.991 | 7020436 | −36.24 | 23.92 | 217.21 |
| 20 | 11333988 | −39.72 | ||||
| 36 | 20650946 | −43.19 | ||||
| WW-H250 | 4 | y = −2369.4x + 24.5 R2 = 0.998 | 8141606 | −36.67 | 19.70 | 203.50 |
| 20 | 13427161 | −39.93 | ||||
| 36 | 19719331 | −43.18 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saha, N.; Volpe, M.; Fiori, L.; Volpe, R.; Messineo, A.; Reza, M.T. Cationic Dye Adsorption on Hydrochars of Winery and Citrus Juice Industries Residues: Performance, Mechanism, and Thermodynamics. Energies 2020, 13, 4686. https://doi.org/10.3390/en13184686
Saha N, Volpe M, Fiori L, Volpe R, Messineo A, Reza MT. Cationic Dye Adsorption on Hydrochars of Winery and Citrus Juice Industries Residues: Performance, Mechanism, and Thermodynamics. Energies. 2020; 13(18):4686. https://doi.org/10.3390/en13184686
Chicago/Turabian StyleSaha, Nepu, Maurizio Volpe, Luca Fiori, Roberto Volpe, Antonio Messineo, and M. Toufiq Reza. 2020. "Cationic Dye Adsorption on Hydrochars of Winery and Citrus Juice Industries Residues: Performance, Mechanism, and Thermodynamics" Energies 13, no. 18: 4686. https://doi.org/10.3390/en13184686
APA StyleSaha, N., Volpe, M., Fiori, L., Volpe, R., Messineo, A., & Reza, M. T. (2020). Cationic Dye Adsorption on Hydrochars of Winery and Citrus Juice Industries Residues: Performance, Mechanism, and Thermodynamics. Energies, 13(18), 4686. https://doi.org/10.3390/en13184686