Effect of Oxidants on Syngas Synthesis from Biogas over 3 wt % Ni-Ce-MgO-ZrO2/Al2O3 Catalyst
Abstract
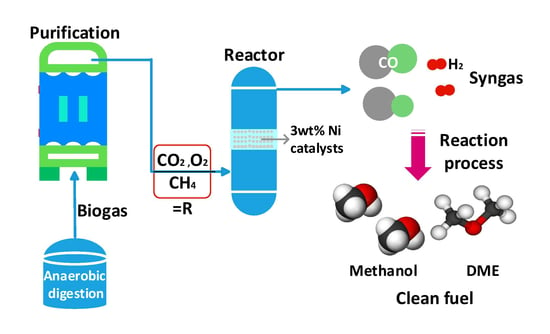
:1. Introduction
2. Biogas Reforming Reaction
2.1. Reaction Mechanism
2.2. Reaction Simulation
2.3. Research Overview
3. Catalyst and Reaction Experiment
3.1. Experimental Apparatus and Method
3.2. Catalyst Preparation and Analysis of Properties
4. Result and Discussion
4.1. Effect of Temperature on CH4 and CO2 Conversion Rates
4.2. Effect of R Value on CH4 and CO2 Conversion Rates
4.3. Effect of R Values on H2 and CO Yields
4.4. Effect of Steam on CH4 and CO2 Conversion Rates
4.5. Synthesis of Chemicals with the Value of H2/CO Ratio
4.6. Durability and Activity of Catalyst
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- UN, United Nations Framework Convention on Climate Change (UNFCCC). Adoption of the Paris Agreement; Report No. FCCC; UN: Rio de Janeiro, Brazil; New York, NY, USA, 2015. [Google Scholar]
- Rogelj, J.; den Elzen, M.; Hohne, N.; Fransen, T.; Fekete, H.; Winkler, H.; Schaeffer, R.; Sha, F.; Riahi, K.; Meinshausen, M. Paris Agreement climate proposals need a boost to keep warming well below 2 °C. Nature 2016, 534, 631–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Energy Agency (IEA) Report, Energy Technology Perspectives 2017. USA. 2017. Available online: www.iea.org (accessed on 4 January 2020).
- Sternberg, A.; Jensa, C.M.; Bardow, A. Life cycle assessment of CO2-based C1-chemicals. Green Chem. 2017, 19, 2244–2259. [Google Scholar] [CrossRef]
- Sternberg, A.; Jensa, C.M.; Bardow, A. Effect of preparation methods on the performance of Co/Al2O3 catalysts for dry reforming of methane. Green Chem. 2014, 2, 885–896. [Google Scholar] [CrossRef]
- Pakhare, D.; Spivey, J. A review of dry (CO2) reforming of methane over noble metal catalysts. J. Chem. Soc. Rev. 2014, 43, 7813–7837. [Google Scholar] [CrossRef] [PubMed]
- Appari, S.; Janardhanan, V.M.; Bauri, R.; Jayanti, S.; Deutschmann, O. A detailed Kinetic model for biogas steam reforming on Ni and catalyst deactivation due to sulfur poisoning. Appl. Catal. A Gen. 2014, 471, 118–125. [Google Scholar] [CrossRef] [Green Version]
- Ashcroft, A.T.; Cheetham, A.K.; Green, M.L.H.; Vernon, P.D.F. Partial oxidation of methane to synthesis gas using carbon dioxide. Nature 1991, 352, 25–226. [Google Scholar] [CrossRef]
- Rostrupnielsen, J.R.; Hansen, J.H.B. CO2-Reforming of methane over Transition Metals. J. Catal. 1993, 144, 38–49. [Google Scholar] [CrossRef]
- Li, H.; Wang, J. Study on CO2 reforming of methane to syngas over Al2O3-ZrO2 supported Ni catalysts prepared via a direct sol-gel process. J. Chem. Eng. Sci. 2004, 59, 4861–4867. [Google Scholar] [CrossRef]
- Sengupta, S.; Deo, G. Modifying alumina with CaO or MgO in supported Ni and Ni-Co catalysts and its effect on dry reforming of CH4. J. CO2 Util. 2015, 10, 62–77. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Uphade, B.S.; Mamman, A.S. Large enhancement in methane-to-syngas conversion activity of supported Ni catalysts due to precoating of catalyst supports with MgO, CaO or rare-earth oxide. Catal. Lett. 1995, 32, 387–390. [Google Scholar] [CrossRef]
- Trutchetti, L.; Murmura, M.A.; Monteleone, G.; Giaconia, A.; Lemonidou, A.A.; Angelic, S.D.; Palma, V.; Ruocco, C.; Annesini, M.C. Kinetic assessment of Ni-based catalysts in low-temperature methane/biogas steam reforming. Int. J. Hydrogen Energy 2016, 41, 16865–16877. [Google Scholar] [CrossRef]
- Sunarno, R.; Panut, M.; Muhammad, A.; Arief, B. Kinetic study catalytic cracking of Bio-oil over silica alumina catalyst. Bioresources 2018, 13, 1917–1929. [Google Scholar] [CrossRef] [Green Version]
- Goula, M.A.; Charisiou, N.D.; Siakavelas, G.; Tzounis, L.; Tsiaoussis, I.; Panagiotopoulou, P.; Goula, G.; Yentekakis, I.V. Syngas production via the biogas dry reforming reaction over Ni supported on zirconia modified with CeO2 or La2O3 catalysts. Int. J. Hydrog. Energy 2017, 42, 13724–13740. [Google Scholar] [CrossRef]
- Halabi, M.H.; de Croon, J.M.; van der Schaaf, J.; Cobden, P.D.; Schouten, J.C. Modeling and analysis of auto thermal reforming of methane to hydrogen in a fixed bed reformer. Chem. Eng. J. 2008, 137, 568–578. [Google Scholar] [CrossRef]
- Jang, W.J.; Shim, J.O.; Kim, H.M.; Yoo, S.Y.; Roh, H.S. A review on dry reforming of methane in aspect of catalytic properties. Catal. Today 2019, 324, 15–26. [Google Scholar] [CrossRef]
- Wang, S.; Lu, G.Q.; Millar, G.J. Carbon Dioxide Reforming of Methane to produce synthesis gas over Metal-supported catalysts: State of the Art. Energy Fuels 1996, 10, 896–904. [Google Scholar] [CrossRef]
- Jang, W.J.; Jeong, D.W.; Shim, J.O.; Kim, H.M.; Roh, H.S.; Son, I.H.; Lee, S.J. Combined steam and carbon dioxide reforming of methane and side reactions: Thermodynamic equilibrium analysis and experimental application. Appl. Energy 2016, 173, 80–91. [Google Scholar] [CrossRef]
- Avraam, D.G.; Halkides, T.I.; Liguras, D.K.; Bereketidou, O.A.; Goula, M.A. An experimental and theoretical approach for the biogas steam reforming reaction. Int. J. Hydrog. Energy 2010, 35, 9818–9827. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Zhang, X.; Mi, Z. Thermodynamic analysis of autothermal steam and CO2 reforming of methane. Int. J. Hydrog. Energy 2008, 33, 2507–2514. [Google Scholar] [CrossRef]
- Amin, N.A.S.; Yaw, T.C. Thermodynamic equilibrium analysis of combined carbon dioxide reforming with partial oxidation of methane to syngas. Int. J. Hydrog. Energy 2007, 32, 1789–1798. [Google Scholar] [CrossRef]
- LeValley, T.L.; Richard, A.R.; Fan, M. The progress in water gas shift and steam reforming hydrogen production technologies—A review. J. Hydrog. Energy 2014, 39, 16983–17000. [Google Scholar] [CrossRef]
- Huang, C.; T-Raissi, A. Thermodynamic analyses of hydrogen production from sub-quality natural gas. Part II: Steam reforming and auto thermal steam reforming. J. Power Sources 2007, 163, 637–644. [Google Scholar] [CrossRef]
- Han, D.B.; Baek, Y.S. A simulation study on the synthesis gas from the reforming reaction of biogas. Trans. Korean Hydrog. New Energy Soc. 2018, 29, 1–10. [Google Scholar] [CrossRef]
- Istadi, I.; Amin, N.A.S. Co-generation of C2 hydrocarbons and synthesis gases from methane and carbon dioxide: A thermodynamic analysis. J. Nat. Gas Chem. 2005, 14, 140–150. [Google Scholar]
- Nikoo, M.K.; Amin, N.A.S. Thermodynamic analysis of carbon dioxide reforming of methane in view of solid carbon formation. Fuel Process. Technol. 2011, 92, 678–691. [Google Scholar] [CrossRef] [Green Version]
- Kho, D.H.; Cho, W.S.; Baek, Y.S. A study on the reaction optimization for the utilization of CO2 and CH4 from Bio-gas. Trans. Korean Hydrog. New Energy Soc. 2016, 27, 554–561. [Google Scholar] [CrossRef] [Green Version]
- Vernon, P.D.F.; Green, M.L.H.; Cheetham, A.K.; Ashcroft, A.T. Partial oxidation of methane to synthesis gas. Catal. Lett. 1990, 6, 181–186. [Google Scholar] [CrossRef]
- Roh, H.S.; Jun, K.W.; Baek, S.C.; Park, S.E. Highly active stable All-Round catalyst for methane reforming reactions: Ni/Ce-ZrO2/θ-Al2O3. Korean Chem. Soc. 2002, 23, 793. [Google Scholar] [CrossRef] [Green Version]
- Youn, B.H.; Shin, B.C.; Lee, C.H.; Lee, J.K.; Jeon, Y.H.; Lee, K.P.; Cho, K.M.; Kim, J.H. Development of Landfill Gas Control and Utilization Technologies. Minist. Environ. Rep. 1997, 1–435. [Google Scholar]
- Cho, W.S.; Choi, K.D.; Baek, Y.S. A effect of reaction conditions on syngas yield for the preparation of syngas from Landfill Gas. Trans. Korean Hydrog. New Energy Soc. 2015, 26, 477–483. [Google Scholar] [CrossRef] [Green Version]
- Izquierdo, U.; Barrio, V.L.; Requies, J.; Cambra, J.F.; Cuemez, M.B.; Arias, P.L. Tri reforming: A new biogas process for synthesis gas and hydrogen production. Int. J. Hydrog. Energy 2013, 38, 7623–7631. [Google Scholar] [CrossRef]
- Bartholomew, C.H. Mechanisms of catalyst deactivation. Appl. Catal. A Gen. 2001, 212, 17–60. [Google Scholar] [CrossRef]
- Choi, K.D. A Study on the Utilization and Characterization of Methane Reforming Reactions over Ni-Catalyst from Landfill Gas (LFG); University of Suwon: Gyeonggi Province, Korea, 2015. [Google Scholar]
- Khalesi, A.; Arandiyan, H.R.; Parvari, M. Effects of lanthanum substitution by strontium and calcium in La–Ni–Al perovskite oxides in dry reforming of methane. Chin. J. Catal. 2008, 29, 960–968. [Google Scholar] [CrossRef]
- Muradov, N.; Smith, F.; T-Raissi, A. Hydrogen production by catalytic processing of renewable methane-rich gases. Int. J. Hydrog. Energy 2008, 33, 2023–2035. [Google Scholar] [CrossRef]
- Song, C.S.; Pan, W. Tri-reforming of methane: A novel concept for synthesis of industrially useful synthesis gas with desired H2/CO ratio using CO2 in fuel gas of power plants without CO2 separation. Am. Chem. Soc. Div. Fuel Chem. 2004, 1, 128–131. [Google Scholar] [CrossRef]















| No. | Reaction | ΔH298 (kJ/mol) |
|---|---|---|
| 1 | CH4 + CO2 ↔ 2CO + 2H2 | 247.0 |
| 2 | CO2 + H2 ↔ CO + H2O | 41.0 |
| 3 | CO + 3H2 ↔ CH4 + H2O | −206.2 |
| 4 | CO2 + 4H2 ↔ CH4 + 2H2O | 165.0 |
| 5 | 2CO ↔ C + CO2 | −172.4 |
| 6 | C + 1/2O2 ↔ CO | 110.0 |
| 7 | C + H2O ↔ CO + H2 | 131.0 |
| 8 | CH4 ↔ C + 2H2 | 74.9 |
| 9 | CH4 + 1/2O2 ↔ CO + H2 | −36.0 |
| 10 | CH4 + H2O ↔ CO + 3H2 | 206.2 |
| 11 | CH4 + 2H2O ↔ CO + 4H2 | 164.9 |
| 12 | CO + H2O ↔ CO2 + H2 | −41.2 |
| 13 | CO + 1/2O2 ↔ CO2 | −283.0 |
| 14 | H2 + 1/2O2 ↔ H2O | −241.8 |
| Condition | Component | |||
|---|---|---|---|---|
| R Value | CH4 (mL/min) | CO2 (mL/min) | O2 (mL/min) | Steam (mL/min) |
| 0.6 | 120 | 60 | 12 | 125 |
| 0.7 | 120 | 60 | 24 | |
| 0.9 | 120 | 60 | 48 | |
| BET (m2/g) | Pore Size (Å) | |
|---|---|---|
| Fresh | 2.94 | 204 |
| After | 2.8 | 193 |
| Metal | Ce | MgO | NiO | Zr |
|---|---|---|---|---|
| Before (wt %) | 1.3 | 3.1 | 3.5 | 2.6 |
| After (wt %) | 0.8 | 1.7 | 3.7 | 2.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, D.; Kim, Y.; Cho, W.; Baek, Y. Effect of Oxidants on Syngas Synthesis from Biogas over 3 wt % Ni-Ce-MgO-ZrO2/Al2O3 Catalyst. Energies 2020, 13, 297. https://doi.org/10.3390/en13020297
Han D, Kim Y, Cho W, Baek Y. Effect of Oxidants on Syngas Synthesis from Biogas over 3 wt % Ni-Ce-MgO-ZrO2/Al2O3 Catalyst. Energies. 2020; 13(2):297. https://doi.org/10.3390/en13020297
Chicago/Turabian StyleHan, Danbee, Yunji Kim, Wonjun Cho, and Youngsoon Baek. 2020. "Effect of Oxidants on Syngas Synthesis from Biogas over 3 wt % Ni-Ce-MgO-ZrO2/Al2O3 Catalyst" Energies 13, no. 2: 297. https://doi.org/10.3390/en13020297
APA StyleHan, D., Kim, Y., Cho, W., & Baek, Y. (2020). Effect of Oxidants on Syngas Synthesis from Biogas over 3 wt % Ni-Ce-MgO-ZrO2/Al2O3 Catalyst. Energies, 13(2), 297. https://doi.org/10.3390/en13020297