Investigation of Dilution Effect on CH4/Air Premixed Turbulent Flame Using OH and CH2O Planar Laser-Induced Fluorescence
Abstract
:1. Introduction
2. Experiment Setup
3. Results and Discussions
3.1. Emission Spectra Analysis
3.2. Image Process
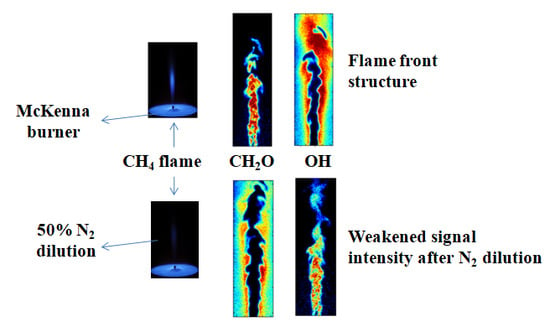
3.3. Analysis of PLIF Images
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Duwig, C.; Li, B.; Li, Z.S.; Aldén, M. High resolution imaging of flameless and distributed turbulent combustion. Combust. Flame 2012, 159, 306–316. [Google Scholar] [CrossRef]
- Medwell, P.R.; Kalt, P.A.; Dally, B.B. Simultaneous imaging of OH, formaldehyde, and temperature of turbulent nonpremixed jet flames in a heated and diluted coflow. Combust. Flame 2007, 148, 48–61. [Google Scholar] [CrossRef]
- Kobayashi, H.; Hagiwara, H.; Kaneko, H.; Ogami, Y. Effects of CO2 dilution on turbulent premixed flames at high pressure and high temperature. Proc. Combust. Inst. 2007, 31, 1451–1458. [Google Scholar] [CrossRef]
- Yoo, H.; Park, B.Y.; Cho, H.; Park, J. Performance Optimization of a Diesel Engine with a Two-Stage Turbocharging System and Dual-Loop EGR Using Multi-Objective Pareto Optimization Based on Diesel Cycle Simulation. Energies 2019, 12, 4223. [Google Scholar] [CrossRef] [Green Version]
- Pramanik, S.; Ravikrishna, R.V. Effect of N2 dilution and preheat temperature on combustion dynamics of syngas in a reverse-flow combustor. Exp. Therm. Fluid Sci. 2020, 110, 109926. [Google Scholar] [CrossRef]
- Karimi, H.; Mardani, A. Investigation of fuel dilution in ethanol spray MILD combustion. Appl. Therm. Eng. 2019, 159, 113898. [Google Scholar]
- Yang, Z.; Yu, X.; Peng, J.; Wang, L.; Dong, Z.; Li, X.; Sun, S.; Meng, S.; Xu, H. Effects of N2, CO2 and H2O dilutions on temperature and concentration fields of OH in methane Bunsen flames by using PLIF thermometry and bi-directional PLIF. Exp. Therm. Fluid Sci. 2017, 81, 209–222. [Google Scholar] [CrossRef]
- Abbasi-Atibeh, E.; Bergthorson, J.M. The effects of differential diffusion in counter-flow premixed flames with dilution and hydrogen enrichment. Combust. Flame 2019, 209, 337–352. [Google Scholar] [CrossRef]
- Wang, D.; Ji, C.; Wang, S.; Meng, H.; Yang, J. Chemical effects of CO2 dilution on CH4 and H2spherical flame. Energy 2019, 185, 316–326. [Google Scholar] [CrossRef]
- Song, Z.; Zhang, X.; Hou, X.; Hu, S. Relationship of the combustion characteristics of natural gas-hydrogen/carbon dioxide mixtures with the ion current and pressure parameters. J. Energy Inst. 2019, 92, 1014–1022. [Google Scholar] [CrossRef]
- Han, Y.; Cai, G.; Wang, H.; Bruno, R.; Abdelkrim, B. Flow characterization and dilution effects of N2 and CO2 on premixed CH4/air flames in a swirl-stabilized combustor. Chin. Phys. B 2014, 3, 386–399. [Google Scholar]
- Lee, M.C.; Seo, S.B.; Yoon, J.; Kim, M.; Yoon, Y. Experimental study on the effect of N2, CO2, and steam dilution on the combustion performance of H2 and CO synthetic gas in an industrial gas turbine. Fuel 2012, 102, 431–438. [Google Scholar] [CrossRef]
- Jourdaine, P.; Mirat, C.; Caudal, J.; Lo, A.; Schuller, T. A comparison between the stabilization of premixed swirling CO2-diluted methane oxy-flames and methane/air flames. Fuel 2016, 201, 431–438. [Google Scholar]
- Xie, Y.; Wang, X.; Wang, J.; Huang, Z. Explosion behavior predictions of syngas/air mixtures with dilutions at elevated pressures: Explosion and intrinsic flame instability parameters. Fuel 2019, 255, 115724. [Google Scholar] [CrossRef]
- Sahu, A.; Wang, C.; Jiang, C.; Xu, H.; Ma, X.; Xu, C.; Bao, X. Effect of CO2 and N2 dilution on laminar premixed MTHF/air flames: Experiments and kinetic studies. Fuel 2019, 255, 115659. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, Z.; Huang, Z.; Hu, E.; Tang, C.; Wang, J. Numerical study on combustion of diluted methanol-air premixed mixtures. Chin. Sci. Bull. 2010, 55, 882–889. [Google Scholar] [CrossRef]
- Wang, Z.H.; Li, B.; Ehn, A.; Sun, Z.W.; Li, Z.S.; Bood, J.; Aldén, M.; Cen, K.F. Investigation of flue-gas treatment with O3 injection using NO and NO2 planar laser-induced fluorescence. Fuel 2010, 89, 2346–2352. [Google Scholar] [CrossRef]
- Luque, J.; Jeffries, J.B.; Smith, G.P.; Crosley, D.R. Quasi-simultaneous detection of CH2O and CH by cavity ring-down absorption and laser-induced fluorescence in a methane/air low-pressure flame. Appl. Phys. B 2001, 73, 731–738. [Google Scholar] [CrossRef]
- Elbaz, A.M.; Roberts, W.L. Experimental study of the inverse diffusion flame using high repetition rate OH/acetone PLIF and PIV. Fuel 2016, 165, 447–461. [Google Scholar] [CrossRef] [Green Version]
- Johchi, A.; Naka, Y.; Shimura, M.; Tanahashi, M.; Miyauchi, T. Investigation on rapid consumption of fine scale unburned mixture islands in turbulent flame via 10 kHz simultaneous CH-OH PLIF and SPIV. Proc. Combust. Inst. 2015, 3, 3663–3671. [Google Scholar] [CrossRef]
- Coriton, B.; Steinberg, A.M.; Frank, J.H. High-speed tomographic PIV and OH PLIF measurements in turbulent reactive flows. Exp. Fluids 2014, 55, 1743. [Google Scholar] [CrossRef]
- Wellander, R.; Richter, M.; Aldén, M. Time-resolved (kHz) 3D imaging of OH PLIF in a flame. Exp. Fluids 2014, 55, 1764. [Google Scholar] [CrossRef]
- Li, H.; Li, B.; Gao, Q.; Zhang, D.; Li, X.; Li, Z. Plane laser-Induced fluorescence for flame surface density calculation of OH/CH2O:A comparative study. J. Combust. Sci. Technol. 2018, 24, 523–527. [Google Scholar]
- Zhu, J.; Zhao, G.; Long, T.; Sun, M.; Li, Q.; Liang, J. Simutaneous OH and CH2O PLIF imaging of flame structures. J. Exp. Fluid Mech. 2016, 30, 55–60. [Google Scholar]
- Yan, H.; Zhang, S.; Xu, X.; Li, F.; Lin, X. Flame structure and dynamics characters investigation by OH and CH2O planar laser-induced fluorescence in the swirl combustor. J. Aerosp. Power 2019, 34, 894–907. [Google Scholar]
- Li, X.; Li, H.; He, K.; Lin, H.; Cheng, L. Analysis of characteristic spectrum of CN/CH/CHO/CH2O/NCN in induction period of methane explosion. J. Chin. Coal Soc. 2014, 39, 2042–2046. [Google Scholar]
- Wang, Z.; Stamatoglou, P.; Zhou, B.; Aldén, M.; Bai, X.S.; Richter, M. Mattias Richtera Investigation of OH and CH2O distributions at ultra-high repetition rates by planar laser induced fluorescence imaging in highly turbulent jet flames. Fuel 2018, 234, 1528–1540. [Google Scholar] [CrossRef]
- Alzueta, M.U.; Glarborg, P. Formation and destruction of CH2O in the exhaust system of a gas engine. Environ. Sci. Technol. 2003, 37, 4512–4516. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Davidson, D.F.; Hanson, R.K. High-temperature laser absorption diagnostics for CH2O and CH3CHO and their application to shock tube kinetic studies. Combust. Flame 2013, 160, 1930–1938. [Google Scholar] [CrossRef]
- Schroeder, J.R.; Crawford, J.H.; Fried, A.; Walega, J.; Weinheimer, A.; Wisthaler, A.; Müller, M.; Mikoviny, T.; Chen, G.; Shook, M.; et al. New insights into the column CH2O/NO2 ratio as an indicator of near-surface ozone sensitivity. J. Geophys. Res. Atmos. 2017, 122, 8885–8907. [Google Scholar] [CrossRef] [Green Version]
- Osborne, J.R.; Ramji, S.A.; Carter, C.D.; Peltier, S.; Hammack, S.; Lee, T.; Steinberg, A.M. Simultaneous 10 kHz TPIV, OH PLIF, and CH2O PLIF measurements of turbulent flame structure and dynamics. Exp. Fluids 2016, 57, 65. [Google Scholar] [CrossRef]
- Hammack, S.D.; Carter, C.D.; Skiba, A.W.; Fugger, C.A.; Felver, J.J.; Miller, J.D.; Gord, J.R.; Lee, T. 20kHz CH2O and OH PLIF with stereo PIV. Opt. Lett. 2018, 5, 1115–1118. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wang, Z.; Jiang, C.; Jiang, Y.; Xu, H.; Wang, J. An optical study of in-cylinder CH2O and OH chemiluminescence in flame-induced reaction front propagation using high speed imaging. Fuel 2014, 134, 603–610. [Google Scholar] [CrossRef]
- Dally, B.B.; Karpetis, A.N.; Barlow, R.S. Structure of turbulent non-premixed jet flames in a diluted hot coflow. Proc. Combust. Inst. 2002, 29, 1147–1154. [Google Scholar] [CrossRef]
- Wang, J.; Matsuno, F.; Okuyama, M.; Ogami, Y.; Kobayashi, H.; Huang, Z. Flame front characteristics of turbulent premixed flames diluted with CO2 and H2O at high pressure and high temperature. Proc. Combust. Inst. 2013, 34, 1429–1436. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Z.; Zhu, Y.; Li, Z.; Zhou, J.; Huang, Z.; Cen, K. Premixed jet flame characteristics of syngas using OH planar laser induced fluorescence. Chin. Sci. Bull. 2011, 56, 2862–2868. [Google Scholar] [CrossRef] [Green Version]
- Weng, W.B.; Wang, Z.H.; He, Y.; Whiddon, R.; Zhou, Y.J.; Li, Z.S.; Cen, K.F. Effect of N2/CO2 dilution on laminar burning velocity of H2-CO-O2 oxy-fuel premixed flame. Int. J. Hydrog. Energy 2015, 40, 1203–1211. [Google Scholar] [CrossRef]
- Wang, Z.H.; Weng, W.B.; He, Y.; Li, Z.S.; Cen, K.F. Effect of H2/CO ratio and N2/CO2 dilution rate on laminar burning velocity of syngas investigated by direct measurement and simulation. Fuel 2015, 141, 285–292. [Google Scholar] [CrossRef]
- Roy, R.; Gupta, A.K. Flame structure and emission signature in distributed combustion. Fuel 2020, 262, 116460. [Google Scholar] [CrossRef]









| Experiment Cases | Premixed Mixture Composition | Reynolds Number (Re) | |||
|---|---|---|---|---|---|
| CH4% | Air% | N2% | CO2% | ||
| Flame 1 (φ = 0.4/DN2 = 0%) | 4.0 | 96.0 | 0 | 0 | 6000 |
| Flame 2 (φ = 0.9/DN2 = 0%) | 8.6 | 91.4 | 0 | 0 | 6000 |
| Flame 3 (φ = 0.9/DN2 = 30%) | 6.0 | 64.0 | 30.0 | 0 | 6000 |
| Flame 4 (φ = 0.9/DN2 = 50%) | 4.3 | 45.7 | 50.0 | 0 | 6000 |
| Flame 5 (φ = 0.9/DCO2 = 50%) | 4.3 | 45.7 | 0 | 50 | 6000 |
| Flame 6 (φ = 0.9/DN2 = 50%) | 4.3 | 45.7 | 50.0 | 0 | 3000 |
| Flame 7 (φ = 0.9/DCO2 = 50%) | 4.3 | 45.7 | 0 | 50 | 3000 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Weng, W.; Zhu, Y.; He, Y.; Wang, Z.; Li, Z. Investigation of Dilution Effect on CH4/Air Premixed Turbulent Flame Using OH and CH2O Planar Laser-Induced Fluorescence. Energies 2020, 13, 325. https://doi.org/10.3390/en13020325
Yang L, Weng W, Zhu Y, He Y, Wang Z, Li Z. Investigation of Dilution Effect on CH4/Air Premixed Turbulent Flame Using OH and CH2O Planar Laser-Induced Fluorescence. Energies. 2020; 13(2):325. https://doi.org/10.3390/en13020325
Chicago/Turabian StyleYang, Li, Wubin Weng, Yanqun Zhu, Yong He, Zhihua Wang, and Zhongshan Li. 2020. "Investigation of Dilution Effect on CH4/Air Premixed Turbulent Flame Using OH and CH2O Planar Laser-Induced Fluorescence" Energies 13, no. 2: 325. https://doi.org/10.3390/en13020325
APA StyleYang, L., Weng, W., Zhu, Y., He, Y., Wang, Z., & Li, Z. (2020). Investigation of Dilution Effect on CH4/Air Premixed Turbulent Flame Using OH and CH2O Planar Laser-Induced Fluorescence. Energies, 13(2), 325. https://doi.org/10.3390/en13020325