Structural, Optical and Photocatalytic Characterization of ZnxCd1−xS Solid Solutions Synthetized Using a Simple Ultrasonic Radiation Method
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of CdS and ZnxCd1−xS Solid Solutions
2.2. Characterization Methods
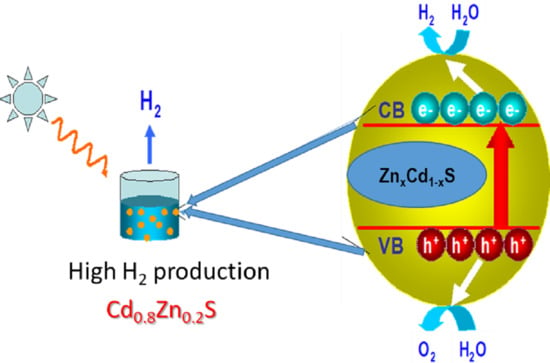
2.3. Photocatalytic Activity
- Photocatalyst + hν → e− (CB) + h+ (VB)
- 2H2O + 2e− (CB) → H2 + 2 OH−
- SO32− + H2O + 2h+ (VB) → SO42− + 2H+
- 2S2− + 2h+ (VB) → S22−
- S22− + SO32− → S2O32− + S2−
- SO32− + S2− + 2h+ (VB) → S2O32−
3. Results and Discussion
3.1. Physicochemical Characterization
3.1.1. XRD Analysis
3.1.2. SEM and TEM Analysis
3.1.3. Raman Spectroscopy Analysis
3.1.4. UV–Vis Diffuse Reflectance Spectra (UV–Vis DRS)
3.2. Photocatalytic Activity–Structure Correlation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.-H.P.; Mielenz, J.R. Renewable Hydrogen Carrier—Carbohydrate: Constructing the Carbon-Neutral Carbohydrate Economy. Energies 2011, 4, 254–275. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhang, Q.; Wang, L.; Li, X.; Huang, W. Interface induce growth of intermediate layer for bandgap engineering insights into photoelectrochemical water splitting. Sci. Rep. 2016, 6, 27241. [Google Scholar] [CrossRef] [Green Version]
- Kimi, M.; Yuliati, L.; Shamsuddin, M. Photocatalytic hydrogen production under visible light over Cd0.1SnxZn0.9−2xS solid solution photocatalysts. Int. J. Hydrog. Energy 2011, 36, 9453–9461. [Google Scholar] [CrossRef]
- Macías-Sánchez, S.; Nava, R.; Hernández-Morales, V.; Acosta-Silva, Y.; Pawelec, B.; Al-Zahrani, S.; Navarro, R.; Fierro, J. Cd1−xZnxS supported on SBA-16 as photocatalysts for water splitting under visible light: Influence of Zn concentration. Int. J. Hydrog. Energy 2013, 38, 11799–11810. [Google Scholar] [CrossRef]
- Maréchal, Y. The Hydrogen Bond and the Water Molecule: The Physics and Chemistry of Water, Aqueous and Bio-Media; Elsevier Science: Amsterdam, The Netherlands, 2007; pp. 311–318. ISBN 9780444519573. [Google Scholar]
- Jacobsson, T.J.; Fjallstrom, V.; Edoff, M.; Edvinsson, T. Sustainable solar hydrogen production: From photoelectrochemical cells to PV-electrolyzers and back again. Energy Environ. Sci. 2014, 7, 2056–2070. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Osterloh, F.E. Inorganic Materials as Catalysts for Photochemical Splitting of Water. Chem. Mater. 2008, 20, 35–54. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nat. Cell Biol. 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, J.; Low, J.; Fang, Y.; Xiao, J.; Chen, X. Engineering heterogeneous semiconductors for solar water splitting. J. Mater. Chem. A 2015, 3, 2485–2534. [Google Scholar] [CrossRef]
- Ji, L.; McDaniel, M.D.; Wang, S.; Posadas, A.B.; Li, X.; Huang, H.; Lee, J.C.; Demkov, A.A.; Bard, A.J.; Ekerdt, J.G.; et al. A silicon-based photocathode for water reduction with an epitaxial SrTiO3 protection layer and a nanostructured catalyst. Nat. Nanotechnol. 2014, 10, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.M.; Aljaber, A.S.; AlQaradawi, S.Y.; Allam, N.K. TiO2 nanotubes with ultrathin walls for enhanced water splitting. Chem. Commun. 2015, 51, 12617–12620. [Google Scholar] [CrossRef] [PubMed]
- Mor, G.K.; Prakasam, H.E.; Varghese, O.K.; Shankar, K.; Grimes, C.A. Vertically Oriented Ti−Fe−O Nanotube Array Films: Toward a Useful Material Architecture for Solar Spectrum Water Photoelectrolysis. Nano Lett. 2007, 7, 2356–2364. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Tang, Y.; Qiu, X.; Yin, J.; Su, C.; Pu, X. Use of synergistic effects of the co-catalyst, p-n heterojunction, and porous structure for improvement of visible-light photocatalytic H2 evolution in porous Ni2O3/Mn0.2Cd0.8S/Cu3P@Cu2S. J. Alloys Compd. 2020, 845, 155569. [Google Scholar] [CrossRef]
- Patidar, D.; Sharma, R.; Jain, N.; Sharma, T.P.; Saxena, N. Optical properties of CdS sintered film. Bull. Mater. Sci. 2006, 29, 21–24. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Gaur, J.K.; Sharma, M.K.; Sharma, T.P. Electrical properties of cadmium telluride screen printed films for photovoltaic application. Chalcogenide Lett. 2008, 5, 171–176. [Google Scholar]
- Li, Q.; Guo, B.; Yu, J.; Ran, J.; Zhang, B.; Yan, H.; Gong, J.R. Highly Efficient Visible-Light-Driven Photocatalytic Hydrogen Production of CdS-Cluster-Decorated Graphene Nanosheets. J. Am. Chem. Soc. 2011, 133, 10878–10884. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Cheng, B.; Yu, J. Hierarchical porous CdS nanosheet-assembled flowers with enhanced visible-light photocatalytic H2-production performance. Appl. Catal. B Environ. 2013, 138-139, 299–303. [Google Scholar] [CrossRef]
- Navarro, R.M.; Sánchez-Sánchez, M.C.; Alvarez-Galvan, M.C.; Del Valle, F.; Fierro, J.L.G. Hydrogen production from renewable sources: Biomass and photocatalytic opportunities. Energy Environ. Sci. 2009, 2, 35–54. [Google Scholar] [CrossRef]
- Joishy, S.; Hebbar, D.N.; Kulkarni, S.D.; Rao, G.K.; Rajendra, B. Band structure controlled solid solution of spray deposited Cd1-x ZnxS films: Investigation on photoluminescence and photo response properties. Phys. B Condens. Matter 2020, 586, 412143. [Google Scholar] [CrossRef]
- Bhatt, A.N.; Verma, U.K.; Kumar, B. Temporal evolution of white light emitting CdS core and Cd1-xZnxS graded shell quantum dots fabricated using single step non-injection technique. Opt. Mater. 2019, 92, 143–149. [Google Scholar] [CrossRef]
- Kozlova, E.A.; Parmon, V.N. Chapter 10. Semiconductor Photocatalysts Based on Nanostructured Cd1−Zn S Solid Solutions in the Reaction of Hydrogen Evolution from Aqueous Solutions of Inorganic Electron Donors under Visible Light. Adv. Nanomater. Catal. Energy 2019, 357–391. [Google Scholar] [CrossRef]
- Ye, Z.; Kong, L.; Chen, F.; Chen, Z.; Lin, Y.; Liu, C. A comparative study of photocatalytic activity of ZnS photocatalyst for degradation of various dyes. Optik 2018, 164, 345–354. [Google Scholar] [CrossRef]
- Kozlova, E.A.; Lyulyukin, M.N.; Markovskaya, D.V.; Selishchev, D.; Cherepanova, S.V.; Kozlov, D.V. Synthesis of Cd1−xZnxS photocatalysts for gas-phase CO2 reduction under visible light. Photochem. Photobiol. Sci. 2019, 18, 871–877. [Google Scholar] [CrossRef]
- Xing, C.; Zhang, Y.; Yan, W.; Guo, L. Band structure-controlled solid solution of Cd1-xCd1-x ZnxSZnxS photocatalyst for hydrogen production by water splitting. Int. J. Hydrog. Energy 2006, 31, 2018–2024. [Google Scholar] [CrossRef]
- Macías-Sánchez, S.; Nava-Mendoza, R.; Hernández-Morales, V.; Acosta-Silva, Y.; Gómez-Herrera, L.; Pawelec, B.; Al-Zahrani, S.M.; Navarro, R.; Fierro, J.L.G. Cd1−xZnxS solid solutions supported on ordered mesoporous silica (SBA-15): Structural features and photocatalytic activity under visible light. Int. J. Hydrog. Energy 2012, 37, 9948–9958. [Google Scholar] [CrossRef]
- Villoria, J.A.; Navarro, R.; Al-Zahrani, S.M.; Fierro, J.L.G.; Al-Zahrani, S.M. Photocatalytic Hydrogen Production on Cd1−xZnxS Solid Solutions under Visible Light: Influence of Thermal Treatment. Ind. Eng. Chem. Res. 2010, 49, 6854–6861. [Google Scholar] [CrossRef]
- Del Valle, F.; Ishikawa, A.; Domen, K.; De La Mano, J.V.; Sanchez-Sanchez, M.; González, I.; Herreras, S.; Mota, N.; Rivas, M.; Álvarez-Galván, M.C. Influence of Zn concentration in the activity of Cd1−xZnxS solid solutions for water splitting under visible light. Catal. Today 2009, 143, 51–56. [Google Scholar] [CrossRef]
- Sasikala, G.; Dhanasekaran, R.; Subramanian, C. Electrodeposition and optical characterisation of CdS thin films on ITO-coated glass. Thin Solid Films 1997, 302, 71–76. [Google Scholar] [CrossRef]
- Singh, V.P. Nano-structured CdTe, CdS and TiO2 for thin film solar cell applications. Sol. Energy Mater. Sol. Cells 2004, 82, 315–330. [Google Scholar] [CrossRef]
- Yavuz, N.; Yuksel, S.A.; Karsli, A.; Günes, S. Inverted structure hybrid solar cells using CdS thin films. Sol. Energy Mater. Sol. Cells 2013, 116, 224–230. [Google Scholar] [CrossRef]
- Mahmood, W.; Shah, N.A. CdZnS thin films sublimated by closed space using mechanical mixing: A new approach. Opt. Mater. 2014, 36, 1449–1453. [Google Scholar] [CrossRef]
- Mooney, J.B.; Radding, S.B. Spray Pyrolysis Processing. Annu. Rev. Mater. Res. 1982, 12, 81–101. [Google Scholar] [CrossRef]
- Ma, Y.Y.; Bube, R.H. Properties of CdS Films Prepared by Spray Pyrolysis. J. Electrochem. Soc. 1977, 124, 1430–1435. [Google Scholar] [CrossRef]
- Baykul, M.; Orhan, N. Band alignment of Cd(1−x)ZnxS produced by spray pyrolysis method. Thin Solid Films 2010, 518, 1925–1928. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, Y.; Qian, H.; Li, Z.; Chen, J. Coating Colloidal Carbon Spheres with CdS Nanoparticles: Microwave-Assisted Synthesis and Enhanced Photocatalytic Activity. Langmuir 2010, 26, 18570–18575. [Google Scholar] [CrossRef] [PubMed]
- Gedanken, A. Using sonochemistry for the fabrication of nanomaterials. Ultrason. Sonochem. 2004, 11, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Suslick, K.S. Sonochemistry. Science 1990, 247, 1439–1445. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Chen, W.; Liang, C.; Wang, Y.; Meng, G.; Zhang, L. Preparation and characterization of CdS nanoparticles by ultrasonic irradiation. Inorg. Chem. Commun. 2001, 4, 208–210. [Google Scholar] [CrossRef]
- Ghows, N.; Entezari, M. A novel method for the synthesis of CdS nanoparticles without surfactant. Ultrason. Sonochem. 2011, 18, 269–275. [Google Scholar] [CrossRef]
- Zak, A.K.; Majid, W.A.; Abrishami, M.E.; Yousefi, R. X-ray analysis of ZnO nanoparticles by Williamson–Hall and size–strain plot methods. Solid State Sci. 2011, 13, 251–256. [Google Scholar] [CrossRef]
- Kubelka, P. New Contributions to the Optics of Intensely Light-Scattering Materials Part I. J. Opt. Soc. Am. 1948, 38, 448. [Google Scholar] [CrossRef]
- Borja-Urby, R.; Diaz-Torres, L.A.; Garcia-Martinez, I.; Uribe, D.B.; Casillas, G.; Ponce, A.; Jose-Yacaman, M. Crystalline and narrow band gap semiconductor BaZrO3: Bi–Si synthesized by microwave–hydrothermal synthesis. Catal. Today 2015, 250, 95–101. [Google Scholar] [CrossRef]
- Munir, S.; Shah, S.M.; Hussain, H.; Khan, R.A. Effect of carrier concentration on the optical band gap of TiO2 nanoparticles. Mater. Des. 2016, 92, 64–72. [Google Scholar] [CrossRef]
- Barajas-Ledesma, E.; García-Benjume, M.; Espitia-Cabrera, I.; Ortiz-Gutiérrez, M.; Espinoza-Beltrán, F.; Mostaghimi, J.; Contreras-García, M. Determination of the band gap of TiO2–Al2O3 films as a function of processing parameters. Mater. Sci. Eng. B 2010, 174, 71–73. [Google Scholar] [CrossRef]
- Nayeem, A.; Yadaiah, K.; Vajralingam, G.; Mahesh, P.; Nagabhooshanam, M. Structural characterization of co-precipitated Cd1-xZnxS: Cu crystals. Int. J. Mod. Phys. B 2002, 16, 481–496. [Google Scholar] [CrossRef]
- Schüth, F.; Wingen, A.; Sauer, J. Oxide loaded ordered mesoporous oxides for catalytic applications. Microporous Mesoporous Mater. 2001, 44, 465–476. [Google Scholar] [CrossRef]
- Reber, J.F.; Meier, K. Photochemical production of hydrogen with zinc sulfide suspensions. J. Phys. Chem. 1984, 88, 5903–5913. [Google Scholar] [CrossRef]
- International Centre for Diffraction Data, 12 Campus Blvd, Newtown Square, PA 19073, USA, ICSD file #081925. Available online: https://www.icdd.com/ (accessed on 5 October 2020).
- Wang, W.; Zhu, W.; Xu, H. Monodisperse, Mesoporous ZnxCd1−xS Nanoparticles as Stable Visible-Light-Driven Photocatalysts. J. Phys. Chem. C 2008, 112, 16754–16758. [Google Scholar] [CrossRef]
- Environmental Chemistry. Periodic Table of Elements. Available online: https://environmentalchemistry.com/yogi/periodic/ionicradius (accessed on 15 May 2012).
- Ichimura, M.; Furukawa, T.; Shirai, K.; Goto, F. Raman and photoluminescence characterizations of electrochemically deposited ZnxCd1—xS layers. Mater. Lett. 1997, 33, 51–55. [Google Scholar] [CrossRef]
- Patel, N.H. Structural And Magnetic Properties Of Undoped And Mn Doped CdS Nanoparticles Prepared By Chemical Co-Precipitation Method. Adv. Mater. Lett. 2014, 5, 671–677. [Google Scholar] [CrossRef]
- Metin, H.; Sat, F.; Erat, S.; Ari, M. Cadmium Sulfide Thin Films grown by CBD: The effect of thermal annealing on the structural, electrical and optical properties. J. Optoelectron. Adv. Mater. 2008, 10, 2622. [Google Scholar]
- Prabhu, R.R.; Khadar, M.A. Study of optical phonon modes of CdS nanoparticles using Raman spectroscopy. Bull. Mater. Sci. 2008, 31, 511–515. [Google Scholar] [CrossRef]
- Navarro, R.M.; Alvarez-Galván, M.C.; De La Mano, J.A.V.; Al-Zahrani, S.M.; Fierro, J.L.G. A framework for visible-light water splitting. Energy Environ. Sci. 2010, 3, 1865–1882. [Google Scholar] [CrossRef]
- Ramaiah, K.S.; Pilkington, R.; Hill, A.; Tomlinson, R.; Bhatnagar, A.K. Structural and optical investigations on CdS thin films grown by chemical bath technique. Mater. Chem. Phys. 2001, 68, 22–30. [Google Scholar] [CrossRef]
- Sabio, E.M.; Chamousis, R.L.; Browning, N.D.; Osterloh, F.E. Photocatalytic Water Splitting with Suspended Calcium Niobium Oxides: Why Nanoscale is Better than Bulk—A Kinetic Analysis. J. Phys. Chem. C 2012, 116, 3161–3170. [Google Scholar] [CrossRef]
- Li, L.; Yan, J.; Wang, T.; Zhao, Z.-J.; Zhang, J.; Gong, J.; Guan, N. Sub-10 nm rutile titanium dioxide nanoparticles for efficient visible-light-driven photocatalytic hydrogen production. Nat. Commun. 2015, 6, 5881. [Google Scholar] [CrossRef]
- Huang, H.-B.; Fang, Z.-B.; Yu, K.; Lü, J.; Cao, R. Visible-light-driven photocatalytic H2 evolution over CdZnS nanocrystal solid solutions: Interplay of twin structures, sulfur vacancies and sacrificial agents. J. Mater. Chem. A 2020, 8, 3882–3891. [Google Scholar] [CrossRef]













| Catalyst | Crystal Size (nm) | λ (nm) | ΔEbg (eV) |
|---|---|---|---|
| CdS | 4.37 | 570 | 2.21 |
| Zn0.05Cd0.95S | 4.16 | 564 | 2.24 |
| Zn0.1Cd0.9S | 4.12 | 558 | 2.28 |
| Zn0.15Cd0.15S | 3.84 | 549 | 2.32 |
| Zn0.2Cd0.8S | 3.80 | 546 | 2.34 |
| Zn0.25Cd0.75S | 3.72 | 541 | 2.39 |
| Sample | 1-LO (cm−1) | Intensity I1-LO | 2-LO (cm−1) | Intensity I2-LO |
|---|---|---|---|---|
| CdS | 294.61 | 270.29 | 595.12 | 137.74 |
| Zn0.05Cd0.95S | 294.87 | 302.86 | 598.97 | 127.63 |
| Zn0.1Cd0.9S | 300.25 | 274.03 | 602.56 | 114.90 |
| Zn0.15Cd0.15S | 303.84 | 248.20 | 606.15 | 96.93 |
| Zn0.2Cd0.8S | 305.64 | 347.42 | 609.74 | 125.38 |
| Zn0.25Cd0.75S | 296.10 | 344.27 | 603.96 | 123.97 |
| Bulk Zn0.2Cd0.8S | Zn0.2Cd0.8S/SBA-16 | Zn0.2Cd0.8S/SBA-15 | |
|---|---|---|---|
| Reference | This work | [4] | [28] |
| Preparation method | Sonochemical coprecipitation | Thermochemical coprecipitation | Termochemical coprecipitation |
| Morphology | Hierarchical 3D morphology | Ordered 3D cage structure with interconnected micro- and mesopores | Hexagonal 2D array of long 1D uniform tubular channels; mesoporous structure |
| H2 production at 5 h [μmol] | 1045 | 987 | 972 |
| Ebg (eV) | 2.34 | 2.20 | 2.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morelos Medina, L.F.; Nava, R.; Cuán Hernández, M.d.l.Á.; Yáñez Soria, O.S.; Pawelec, B.; Navarro, R.M.; Ornelas Gutiérrez, C.E. Structural, Optical and Photocatalytic Characterization of ZnxCd1−xS Solid Solutions Synthetized Using a Simple Ultrasonic Radiation Method. Energies 2020, 13, 5603. https://doi.org/10.3390/en13215603
Morelos Medina LF, Nava R, Cuán Hernández MdlÁ, Yáñez Soria OS, Pawelec B, Navarro RM, Ornelas Gutiérrez CE. Structural, Optical and Photocatalytic Characterization of ZnxCd1−xS Solid Solutions Synthetized Using a Simple Ultrasonic Radiation Method. Energies. 2020; 13(21):5603. https://doi.org/10.3390/en13215603
Chicago/Turabian StyleMorelos Medina, Luis Fernando, Rufino Nava, María de los Ángeles Cuán Hernández, Omar Said Yáñez Soria, Bárbara Pawelec, Rufino M. Navarro, and Carlos Elías Ornelas Gutiérrez. 2020. "Structural, Optical and Photocatalytic Characterization of ZnxCd1−xS Solid Solutions Synthetized Using a Simple Ultrasonic Radiation Method" Energies 13, no. 21: 5603. https://doi.org/10.3390/en13215603
APA StyleMorelos Medina, L. F., Nava, R., Cuán Hernández, M. d. l. Á., Yáñez Soria, O. S., Pawelec, B., Navarro, R. M., & Ornelas Gutiérrez, C. E. (2020). Structural, Optical and Photocatalytic Characterization of ZnxCd1−xS Solid Solutions Synthetized Using a Simple Ultrasonic Radiation Method. Energies, 13(21), 5603. https://doi.org/10.3390/en13215603