Pawpaw (Carica papaya) Peel Waste as a Novel Green Heterogeneous Catalyst for Moringa Oil Methyl Esters Synthesis: Process Optimization and Kinetic Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Moringa Oil and Other Chemicals
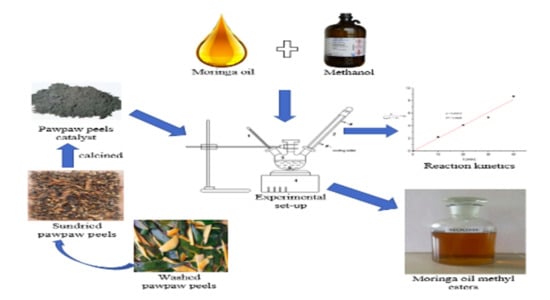
2.2. Methods
Catalyst Preparation
2.3. Catalyst Characterization
2.3.1. Potency Test of Catalyst Developed
2.3.2. Experimental Design and Data Analysis for MOOME Production
2.3.3. Transesterification of MOO to MOOME
2.3.4. Kinetic Modeling
3. Results and Discussion
3.1. Analyses of Catalyst Developed from Pawpaw Peels
3.1.1. SEM/EDX Characterization of CPP
3.1.2. FTIR Analysis of CPP
3.1.3. XRD Analysis of CPP
3.1.4. Physisorption Analysis of CPP
3.2. Regression Model for MOOME Production Process
3.3. Influence of Process Variables on MOOME Yield
3.4. Process Variables Optimization and Model Verification
3.5. Reusability of CPP Catalyst
3.6. MOOME Characterization
3.7. MOOME Production Process Kinetics
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| BET | Brunauer–Emmett–Teller |
| BJH | Brunauer–Joyner–Halenda |
| CPP | Calcined pawpaw peels |
| EDX | Energy-dispersive X-ray spectroscopy |
| FTIR | Fourier transform infrared |
| MOO | Moringa oleifera oil |
| MOOME | Moringa oleifera oil methyl esters |
| R2 | Coefficient of determination |
| RPP | Raw pawpaw peel |
| SEM | Scanning electron microscopy |
| SNR | Signal-to-noise ratio |
| XRD | X-ray diffraction |
References
- Abdullah, S.H.Y.S.; Hanapi, N.H.M.; Azid, A.; Umar, R.; Juahir, H.; Khatoon, H.; Endut, A. A review of biomass-derived heterogeneous catalyst for a sustainable biodiesel production. Renew. Sustain. Energy Rev. 2017, 70, 1040–1051. [Google Scholar] [CrossRef]
- Leung, D.Y.; Wu, X.; Leung, M. A review on biodiesel production using catalyzed transesterification. Appl. Energy 2010, 87, 1083–1095. [Google Scholar] [CrossRef]
- Odude, V.O.; Adesina, A.J.; Oyetunde, O.O.; Adeyemi, O.O.; Ishola, N.B.; Etim, A.O.; Betiku, E. Application of agricultural waste-based catalysts to transesterification of esterified palm kernel oil into biodiesel: A case of banana fruit peel versus cocoa pod husk. Waste and Biomass Valoriz. 2019, 10, 877–888. [Google Scholar] [CrossRef]
- Stamenković, O.S.; Todorović, Z.B.; Lazić, M.L.; Veljković, V.B.; Skala, D.U. Kinetics of sunflower oil methanolysis at low temperatures. Bioresour. Technol. 2008, 99, 1131–1140. [Google Scholar] [CrossRef] [PubMed]
- Likozar, B.; Levec, J. Effect of process conditions on equilibrium, reaction kinetics and mass transfer for triglyceride transesterification to biodiesel: Experimental and modeling based on fatty acid composition. Fuel Process. Technol. 2014, 122, 30–41. [Google Scholar] [CrossRef]
- Negm, N.A.; Sayed, G.H.; Yehia, F.Z.; Habib, O.I.; Mohamed, E.A. Biodiesel production from one-step heterogeneous catalyzed process of castor oil and jatropha oil using novel sulphonated phenyl silane montmorillonite catalyst. J. Mol. Liq. 2017, 234, 157–163. [Google Scholar] [CrossRef]
- Betiku, E.; Etim, A.O.; Pereao, O.; Ojumu, T.V. Two-step conversion of neem (Azadirachta indica) seed oil into fatty methyl esters using an heterogeneous biomass-based catalyst: An example of cocoa pod husk. Energy Fuels 2017, 31, 6182–6193. [Google Scholar] [CrossRef]
- Vadery, V.; Narayanan, B.N.; Ramakrishnan, R.M.; Cherikkallinmel, S.K.; Sugunan, S.; Narayanan, D.P.; Sasidharan, S. Room temperature production of jatropha biodiesel over coconut husk ash. Energy 2014, 70, 588–594. [Google Scholar] [CrossRef]
- Silitonga, A.; Mahlia, T.; Kusumo, F.; Dharma, S.; Sebayang, A.; Sembiring, R.; Shamsuddin, A. Intensification of reutealis trisperma biodiesel production using infrared radiation: Simulation, optimisation and validation. Renew. Energy 2019, 133, 520–527. [Google Scholar] [CrossRef]
- Silitonga, A.; Shamsuddin, A.; Mahlia, T.; Milano, J.; Kusumo, F.; Siswantoro, J.; Dharma, S.; Sebayang, A.; Masjuki, H.; Ong, H.C. Biodiesel synthesis from Ceiba pentandra oil by microwave irradiation-assisted transesterification: ELM modeling and optimization. Renew. Energy 2020, 146, 1278–1291. [Google Scholar] [CrossRef]
- Ong, H.C.; Milano, J.; Silitonga, A.S.; Hassan, M.H.; Wang, C.-T.; Mahlia, T.M.I.; Siswantoro, J.; Kusumo, F.; Sutrisno, J. Biodiesel production from Calophyllum inophyllum-Ceiba pentandra oil mixture: Optimization and characterization. J. Clean. Prod. 2019, 219, 183–198. [Google Scholar] [CrossRef]
- Oraegbunam, J.C.; Oladipo, B.; Falowo, O.A.; Betiku, E. Clean sandbox (Hura crepitans) oil methyl esters synthesis: A kinetic and thermodynamic study through pH monitoring approach. Renew. Energy 2020, 160, 526–537. [Google Scholar]
- Tan, Y.H.; Abdullah, M.O.; Nolasco-Hipolito, C.; Taufiq-Yap, Y.H. Waste ostrich-and chicken-eggshells as heterogeneous base catalyst for biodiesel production from used cooking oil: Catalyst characterization and biodiesel yield performance. Appl. Energy 2015, 160, 58–70. [Google Scholar] [CrossRef]
- Hossain, A.S.; Salleh, A.; Boyce, A.N.; Chowdhury, P.; Naqiuddin, M. Biodiesel fuel production from algae as renewable energy. Am. J. Biochem. Biotechnol. 2008, 4, 250–254. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, A.; Prasad, R. Triglycerides-based diesel fuels. Renew. Sustain. Energy Rev. 2000, 4, 111–133. [Google Scholar] [CrossRef]
- Boro, J.; Thakur, A.J.; Deka, D. Solid oxide derived from waste shells of Turbonilla striatula as a renewable catalyst for biodiesel production. Fuel Process. Technol. 2011, 92, 2061–2067. [Google Scholar] [CrossRef]
- Pinto, A.C.; Guarieiro, L.L.; Rezende, M.J.; Ribeiro, N.M.; Torres, E.A.; Lopes, W.A.; Pereira, P.A.d.P.; Andrade, J.B.d. Biodiesel: An overview. J. Braz. Chem. Soc. 2005, 16, 1313–1330. [Google Scholar] [CrossRef] [Green Version]
- Dhawane, S.H.; Kumar, T.; Halder, G. Biodiesel synthesis from Hevea brasiliensis oil employing carbon supported heterogeneous catalyst: Optimization by Taguchi method. Renew. Energy 2016, 89, 506–514. [Google Scholar] [CrossRef]
- Betiku, E.; Akintunde, A.M.; Ojumu, T.V. Banana peels as a biobase catalyst for fatty acid methyl esters production using napoleon’s plume (Bauhinia monandra) seed oil: A process parameters optimization study. Energy 2016, 103, 797–806. [Google Scholar] [CrossRef]
- Gohain, M.; Devi, A.; Deka, D. Musa balbisiana colla peel as highly effective renewable heterogeneous base catalyst for biodiesel production. Ind. Crops. Prods. 2017, 109, 8–18. [Google Scholar] [CrossRef]
- Pathak, G.; Das, D.; Rajkumari, K.; Rokhum, L. Exploiting waste: Towards a sustainable production of biodiesel using Musa acuminata peel ash as a heterogeneous catalyst. Green Chem. 2018, 20, 2365–2373. [Google Scholar] [CrossRef]
- Onoji, S.E.; Iyuke, S.E.; Igbafe, A.I.; Daramola, M.O. Transesterification of rubber seed oil to biodiesel over a calcined waste rubber seed shell catalyst: Modeling and optimization of process variables. Energy Fuels 2017, 31, 6109–6119. [Google Scholar] [CrossRef]
- Mendonça, I.M.; Paes, O.A.; Maia, P.J.; Souza, M.P.; Almeida, R.A.; Silva, C.C.; Duvoisin, S., Jr.; de Freitas, F.A. New heterogeneous catalyst for biodiesel production from waste tucumã peels (Astrocaryum aculeatum meyer): Parameters optimization study. Renew. Energy 2019, 130, 103–110. [Google Scholar] [CrossRef]
- Zhao, C.; Lv, P.; Yang, L.; Xing, S.; Luo, W.; Wang, Z. Biodiesel synthesis over biochar-based catalyst from biomass waste pomelo peel. Energy Convers. Manag. 2018, 160, 477–485. [Google Scholar] [CrossRef]
- Etim, A.O.; Betiku, E.; Ajala, S.O.; Olaniyi, P.J.; Ojumu, T.V. Potential of ripe plantain fruit peels as an ecofriendly catalyst for biodiesel synthesis: Optimization by artificial neural network integrated with genetic algorithm. Sustainability 2018, 10, 707. [Google Scholar] [CrossRef] [Green Version]
- Medina, J.; Gutiérrez, G.V.; García, H. Pawpaw: Post-harvest Operation. Compendium on Post-harvest Operations. Available online: http://www.fao.org/fileadmin/user_upload/inpho/docs/Post_Harvest_Compendium_-_Pawpaw__Papaya_.pdf (accessed on 15 September 2019).
- Evans, E.A.; Ballen, F.H. An Overview of Global Papaya Production, Trade, and Consumption; University of Florida: Gainesville, FL, USA, 2012. [Google Scholar]
- FAOSTAT. Statistical Databases. Food and Agriculture Organization of the United Nations. Statistics Division 2016. Available online: www.fao.org/faostat/en/#data/QC (accessed on 23 January 2018).
- Gohain, M.; Laskar, K.; Paul, A.K.; Daimary, N.; Maharana, M.; Goswami, I.K.; Hazarika, A.; Bora, U.; Deka, D. Carica papaya stem: A source of versatile heterogeneous catalyst for biodiesel production and c–c bond formation. Renew. Energy 2020, 147, 541–555. [Google Scholar] [CrossRef]
- Oladipo, B.; Betiku, E. Process optimization of solvent extraction of seed oil from Moringa oleifera: An appraisal of quantitative and qualitative process variables on oil quality using d-optimal design. Biocatal. Agric. Biotechnol. 2019, 20, 101187. [Google Scholar] [CrossRef]
- Rashid, U.; Anwar, F.; Moser, B.R.; Knothe, G. Moringa oleifera oil: A possible source of biodiesel. Bioresour. Technol. 2008, 99, 8175–8179. [Google Scholar] [CrossRef]
- Kivevele, T.T.; Mbarawa, M.M.; Bereczky, A.K.; Zoöldy, M.T. Evaluation of the oxidation stability of biodiesel produced from Moringa oleifera oil. Energy Fuels 2011, 25, 5416–5421. [Google Scholar]
- Zubairu, A.; Ibrahim, F.S. Moringa oleifera oilseed as viable feedstock for biodiesel production in northern Nigeria. Int. J. Energy Eng. 2014, 4, 21–25. [Google Scholar]
- Mofijur, M.; Masjuki, H.; Kalam, M.; Rasul, M.; Atabani, A.E.; Hazrat, M.; Mahmudul, H. Effect of biodiesel-diesel blending on physico-chemical properties of biodiesel produced from Moringa oleifera. Proc. Eng. 2015, 105, 665–669. [Google Scholar] [CrossRef]
- Karthickeyan, V. Effect of cetane enhancer on Moringa oleifera biodiesel in a thermal coated direct injection diesel engine. Fuel 2019, 235, 538–550. [Google Scholar] [CrossRef]
- Kafuku, G.; Lam, M.K.; Kansedo, J.; Lee, K.T.; Mbarawa, M. Heterogeneous catalyzed biodiesel production from Moringa oleifera oil. Fuel Process. Technol. 2010, 91, 1525–1529. [Google Scholar] [CrossRef]
- Aziz, M.; Triwahyono, S.; Jalil, A.; Rapai, H.; Atabani, A. Transesterification of Moringa oleifera oil to biodiesel using potassium flouride loaded eggshell as catalyst. Malays. J. Catal. 2016, 1, 22–26. [Google Scholar]
- Niju, S.; Anushya, C.; Balajii, M. Process optimization for biodiesel production from Moringa oleifera oil using conch shells as heterogeneous catalyst. Environ. Prog. Sustain. Energy 2019, 38, e13015. [Google Scholar] [CrossRef]
- Esmaeili, H.; Yeganeh, G.; Esmaeilzadeh, F. Optimization of biodiesel production from Moringa oleifera seeds oil in the presence of nano-mgo using taguchi method. Int. Nano Lett. 2019, 9, 257–263. [Google Scholar] [CrossRef] [Green Version]
- Ighose, B.O.; Adeleke, I.A.; Damos, M.; Junaid, H.A.; Okpalaeke, K.E.; Betiku, E. Optimization of biodiesel production from Thevetia peruviana seed oil by adaptive neuro-fuzzy inference system coupled with genetic algorithm and response surface methodology. Energy Convers. Manag. 2017, 132, 231–240. [Google Scholar] [CrossRef]
- Ye, W.; Gao, Y.; Ding, H.; Liu, M.; Liu, S.; Han, X.; Qi, J. Kinetics of transesterification of palm oil under conventional heating and microwave irradiation, using cao as heterogeneous catalyst. Fuel 2016, 180, 574–579. [Google Scholar] [CrossRef]
- Oladipo, B.; Betiku, E. Optimization and kinetic studies on conversion of rubber seed (Hevea brasiliensis) oil to methyl esters over a green biowaste catalyst. J. Environ. Manag. 2020, 268, 110705. [Google Scholar]
- Hameed, B.H.; Lai, L.; Chin, L. Production of biodiesel from palm oil (Elaeis guineensis) using heterogeneous catalyst: An optimized process. Fuel Process. Technol. 2009, 90, 606–610. [Google Scholar] [CrossRef]
- Kumar, D.; Ali, A. Nanocrystalline k–cao for the transesterification of a variety of feedstocks: Structure, kinetics and catalytic properties. Biomass Bioenergy 2012, 46, 459–468. [Google Scholar] [CrossRef]
- Sarmah, M.; Dewan, A.; Mondal, M.; Thakur, A.J.; Bora, U. Analysis of the water extract of waste papaya bark ash and its implications as an in situ base in the ligand-free recyclable suzuki–miyaura coupling reaction. RSC Adv. 2016, 6, 28981–28985. [Google Scholar] [CrossRef]
- Nath, B.; Kalita, P.; Das, B.; Basumatary, S. Highly efficient renewable heterogeneous base catalyst derived from waste Sesamum indicum plant for synthesis of biodiesel. Renew. Energy 2020, 151, 295–310. [Google Scholar] [CrossRef]
- Betiku, E.; Ajala, S.O. Modeling and optimization of Thevetia peruviana (yellow oleander) oil biodiesel synthesis via Musa paradisiaca (plantain) peels as heterogeneous base catalyst: A case of artificial neural network vs. Response surface methodology. Ind. Crops. Prods. 2014, 53, 314–322. [Google Scholar] [CrossRef]
- Sharma, M.; Khan, A.A.; Puri, S.; Tuli, D. Wood ash as a potential heterogeneous catalyst for biodiesel synthesis. Biomass Bioenergy 2012, 41, 94–106. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry; John Wiley & Sons Ltd: Chichester, UK, 2000; pp. 10815–10837. [Google Scholar]
- Yamaguchi, T.; Wang, Y.; Komatsu, M.; Ookawa, M. Preparation of new solid bases derived from supported metal nitrates and carbonates. Catal. Surv. Jpn. 2002, 5, 81–89. [Google Scholar] [CrossRef]
- Basumatary, S.; Nath, B.; Das, B.; Kalita, P.; Basumatary, B. Utilization of renewable and sustainable basic heterogeneous catalyst from Heteropanax fragrans (Kesseru) for effective synthesis of biodiesel from Jatropha curcas oil. Fuel 2021, 286, 119357. [Google Scholar] [CrossRef]
- Falowo, O.A.; Ojumu, T.V.; Pereao, O.; Betiku, E. Sustainable biodiesel synthesis from honne-rubber-neem oil blend with a novel mesoporous base catalyst synthesized from a mixture of three agrowastes. Catalysts 2020, 10, 190. [Google Scholar] [CrossRef] [Green Version]
- Betiku, E.; Okeleye, A.A.; Ishola, N.B.; Osunleke, A.S.; Ojumu, T.V. Development of a novel mesoporous biocatalyst derived from kola nut pod husk for conversion of kariya seed oil to methyl esters: A case of synthesis, modeling and optimization studies. Catal. Lett. 2019, 149, 1772–1787. [Google Scholar] [CrossRef]
- Falowo, O.A.; Oloko-Oba, M.I.; Betiku, E. Biodiesel production intensification via microwave irradiation-assisted transesterification of oil blend using nanoparticles from elephant-ear tree pod husk as a base heterogeneous catalyst. Chem. Eng. Process. Process Intensif. 2019, 140, 157–170. [Google Scholar] [CrossRef]
- Sing, K.S. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Shan, R.; Chen, G.; Yan, B.; Shi, J.; Liu, C. Porous cao-based catalyst derived from pss-induced mineralization for biodiesel production enhancement. Energy Convers. Manag. 2015, 106, 405–413. [Google Scholar] [CrossRef]
- Myers, R.H.; Montgomery, D.C.; Anderson-Cook, C. Response Surface Methodology: Product and Process Optimization Using Designed Experiments; John Wiley & Sons: New York, NY, USA, 2009. [Google Scholar]
- Knothe, G.; Razon, L.F. Biodiesel fuels. Prog. Energy Combust. Sci. 2017, 58, 36–59. [Google Scholar] [CrossRef]
- Ahmad, J.; Yusup, S.; Bokhari, A.; Kamil, R.N.M. Study of fuel properties of rubber seed oil based biodiesel. Energy Convers. Manag. 2014, 78, 266–275. [Google Scholar] [CrossRef]
- Lv, Y.; Sun, S.; Liu, J. Biodiesel production catalyzed by a methanol-tolerant lipase a from Candida antarctica in the presence of excess water. ACS Omega 2019, 4, 20064–20071. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Guo, Y. Transesterification of neat and used frying oil: Optimization for biodiesel production. Fuel Process. Technol. 2006, 87, 883–890. [Google Scholar] [CrossRef]
- Abu-Jrai, A.M.; Jamil, F.; Ala’a, H.; Baawain, M.; Al-Haj, L.; Al-Hinai, M.; Al-Abri, M.; Rafiq, S. Valorization of waste date pits biomass for biodiesel production in presence of green carbon catalyst. Energy Convers. Manag. 2017, 135, 236–243. [Google Scholar] [CrossRef]
- Miladinović, M.R.; Zdujić, M.V.; Veljović, D.N.; Krstić, J.B.; Banković-Ilić, I.B.; Veljković, V.B.; Stamenković, O.S. Valorization of walnut shell ash as a catalyst for biodiesel production. Renew. Energy 2020, 147, 1033–1043. [Google Scholar] [CrossRef]
- Nath, B.; Das, B.; Kalita, P.; Basumatary, S. Waste to value addition: Utilization of waste Brassica nigra plant derived novel green heterogeneous base catalyst for effective synthesis of biodiesel. J. Clean. Prod. 2019, 239, 118112. [Google Scholar] [CrossRef]
- Krishnamurthy, K.; Sridhara, S.; Kumar, C.A. Optimization and kinetic study of biodiesel production from Hydnocarpus wightiana oil and dairy waste scum using snail shell cao nano catalyst. Renew. Energy 2020, 146, 280–296. [Google Scholar] [CrossRef]
- Laskar, I.B.; Rajkumari, K.; Gupta, R.; Chatterjee, S.; Paul, B.; Rokhum, L. Waste snail shell derived heterogeneous catalyst for biodiesel production by the transesterification of soybean oil. RSC Adv. 2018, 8, 20131–20142. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Zhao, C.; Shan, R.; Wang, Y.; Yuan, H. A novel peat biochar supported catalyst for the transesterification reaction. Energy Convers. Manag. 2017, 139, 89–96. [Google Scholar] [CrossRef]
- Suthisripok, T.; Semsamran, P. The impact of biodiesel b100 on a small agricultural diesel engine. Tribol. Int. 2018, 128, 397–409. [Google Scholar] [CrossRef]
- Wu, M.; Wu, G.; Han, L.; Wang, J. Low-temperature fluidity of biodiesel fuel prepared from edible vegetable oil. Pet. Process. Petrochem. 2005, 36, 57–60. [Google Scholar]
- Verma, P.; Sharma, M.; Dwivedi, G. Evaluation and enhancement of cold flow properties of palm oil and its biodiesel. Energy Rep. 2016, 2, 8–13. [Google Scholar] [CrossRef] [Green Version]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists; AOAC: Washington, DC, USA, 1990. [Google Scholar]
- Demirbaş, A. Fuel properties and calculation of higher heating values of vegetable oils. Fuel 1998, 77, 1117–1120. [Google Scholar] [CrossRef]
- Krisnangkura, K. A simple method for estimation of cetane index of vegetable oil methyl esters. J. Am. Oil Chem. Soc. 1986, 63, 552–553. [Google Scholar] [CrossRef]
- Sharafutdinov, I.; Stratiev, D.; Shishkova, I.; Dinkov, R.; Batchvarov, A.; Petkov, P.; Rudnev, N. Cold flow properties and oxidation stability of blends of near zero sulfur diesel from ural crude oil and fame from different origin. Fuel 2012, 96, 556–567. [Google Scholar] [CrossRef]
- Rashid, U.; Anwar, F.; Ashraf, M.; Saleem, M.; Yusup, S. Application of response surface methodology for optimizing transesterification of Moringa oleifera oil: Biodiesel production. Energy Convers. Manag. 2011, 52, 3034–3042. [Google Scholar] [CrossRef]
- Guillén, M.D.; Cabo, N. Relationships between the composition of edible oils and lard and the ratio of the absorbance of specific bands of their fourier transform infrared spectra. Role of some bands of the fingerprint region. J. Agric. Food Chem. 1998, 46, 1788–1793. [Google Scholar] [CrossRef]
- Stamenković, O.S.; Lazić, M.; Todorović, Z.; Veljković, V.; Skala, D. The effect of agitation intensity on alkali-catalyzed methanolysis of sunflower oil. Bioresour. Technol. 2007, 98, 2688–2699. [Google Scholar] [CrossRef]
- Darnoko, D.; Cheryan, M. Kinetics of palm oil transesterification in a batch reactor. J. Am. Oil Chem. Soc. 2000, 77, 1263–1267. [Google Scholar] [CrossRef]
- Foon, C.S.; May, C.Y.; Ngan, M.A.; Hock, C.C. Kinetics study on transesterification of palm oil. J. Oil Palm Res. 2004, 16, 19–29. [Google Scholar]
- Singh, A.K.; Fernando, S.D. Reaction kinetics of soybean oil transesterification using heterogeneous metal oxide catalysts. Chem. Eng. Technol. 2007, 30, 1716–1720. [Google Scholar] [CrossRef]












| Run Number | Variables and Their Levels | Experimental MOOME Yield (wt.%) | |||
|---|---|---|---|---|---|
| MeOH:MOO Molar Ratio | CPP Loading (wt.%) | Reaction Temperature (°C) | Reaction Time (min) | ||
| 1 | 3 | 2 | 35 | 40 | 91.65 |
| 2 | 15 | 2 | 65 | 60 | 82.4 |
| 3 | 9 | 2 | 50 | 80 | 89.19 |
| 4 | 15 | 5 | 50 | 40 | 83.22 |
| 5 | 15 | 3.5 | 35 | 80 | 84.6 |
| 6 | 9 | 3.5 | 65 | 40 | 96.36 |
| 7 | 3 | 3.5 | 50 | 60 | 94.08 |
| 8 | 9 | 5 | 35 | 60 | 90.58 |
| 9 | 3 | 5 | 65 | 80 | 87.13 |
| Heat (°C) | Composition (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C | O | Mg | P | S | Cl | K | Ca | Fe | Na | Si | |
| RPP | 56.81 | 41.86 | 0.00 | 0.00 | 0.00 | 0.00 | 1.16 | 0.17 | 0.00 | 0.00 | 0.00 |
| 200 | 46.59 | 28.37 | 1.26 | 2.81 | 2.57 | 0.64 | 14.75 | 3.01 | 0.00 | 0.00 | 0.00 |
| 400 | 32.32 | 36.33 | 1.67 | 3.62 | 2.15 | 0.59 | 20.37 | 2.95 | 0.00 | 0.00 | 0.00 |
| 600 | 29.16 | 36.72 | 1.00 | 3.04 | 2.45 | 0.87 | 23.89 | 2.86 | 0.00 | 0.00 | 0.00 |
| 800 | 40.85 | 32.86 | 0.43 | 1.62 | 2.34 | 0.63 | 19.26 | 0.00 | 1.63 | 0.37 | 0.00 |
| 1000 | 18.40 | 46.16 | 0.00 | 2.91 | 2.59 | 0.00 | 24.41 | 3.09 | 0.00 | 0.95 | 1.50 |
| Source | ANOVA for the Model | Accuracy Test | |||||
|---|---|---|---|---|---|---|---|
| SS | df | MS | F-Value | p-Value | Parameter | Value | |
| Model | 188.85 | 6 | 31.47 | 416.82 | 0.0024 | Standard deviation | 0.27 |
| A—MeOH:MOO | 132.73 | 2 | 66.37 | 878.89 | 0.0011 | Mean | 88.80 |
| B—CPP loading | 38.19 | 2 | 19.09 | 252.85 | 0.0039 | %CV | 0.31 |
| D—Reaction time | 17.93 | 2 | 8.97 | 118.73 | 0.0084 | R2 | 0.9992 |
| Residual | 0.15 | 2 | 0.076 | Adjusted R2 | 0.9968 | ||
| Total | 189.00 | 8 | Predicted R2 | 0.9838 | |||
| SNR | 57.604 | ||||||
| Source of Triglyceride | Catalyst | Calcination Temperature (°C), Calcination Time (h) | Catalyst Characterization | Process Optimization Conditions | Reusability Cycle (Yield %) | Biodiesel Yield (wt.%) | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Diameter (nm) | MeOH:Oil Molar Ratio | Reaction Temperature (°C) | Catalyst Loading (wt.%) | Reaction Time (min) | ||||||
| Moringa oil | Pawpaw peels | 600, 4 | 3.6042 | 0.00706 | 8.54 | 9:1 | 35 | 3.5 | 40 | 4 (90.10) | 96.43 | This study |
| Moringa oil | /– | 300, 2 | 13.90 | 0.0403 | 13.7 | 1:19.5 | 150 | 3 | 150 | - | 84 | [36] |
| Moringa oil | KF/eggshell | 820, 4 | 6 | 0.0556 | - | 6:1 | 50 | 5 | 60 | - | 94.2 | [37] |
| Moringa oil | Conch shells | 900, 3 | 1.19 | - | - | 8.6662:1 | 65 | 8.022 | 130 | - | 97.06 | [38] |
| Moringa oil | MgO nanocatalyst | - | 14.19 | 0.045 | - | 12:1 | 45 | 1 | 4 | - | 93.69 | [39] |
| WCO | Carica papaya stem | 700, 4 | 78.681 | 0.349 | 3.2148 | 9:1 | 60 | 2 | 180 | 5 (85) | 95.23 | [29] |
| Kariya oil | Kola nut pod husks | 500, 4 | 5.2199 | 0.0122 | 9.3174 | 6:1 | 65 | 3 | 75 | 4 (96.28) | 98.67 | [53] |
| Sunflower oil | Walnut shell ash | 800, 2 | 8.8 | 0.000075 | <7.5 | 12:1 | 60 | 5 | 10 | 4 | >98 | [63] |
| Soybean oil | Waste Brassica nigra plants | 550, 2 | 7.308 | 0.011 | 1.67 | 12:1 | 65 | 7 | 25 | 3 (96) | 98.79 | [64] |
| Diary waste scum | Waste snail shell | 900, 3.5 | 9.37 | 0.0538 | 2.29 | 12.7:1 | 58.56 | 0.866 | 119.684 | 5 (86.85) | 96.929 | [65] |
| Soybean oil | Banana peels | Open-air burned | 1.4546 | 0.00515 | 14.1628 | 6:1 | RT | 0.7 | 240 | 4 (52.16) | 98.95 | [21] |
| Soybean oil | Waste snail shell | 900, 4 | 7 | 0.0312 | 14.8 | 6:1 | RT | 3 | 420 | 8 (91) | 98 | [66] |
| Jatropha oil | Wood ash | 800, 3 | 3.72 | - | - | 12:1 | 65 | 3 | 180 | - | 97.7 | [48] |
| Palm oil | Solid waste peat | 600, 2 | 20.04 | 0.03155 | - | 8:1 | 65 | 5 | 90 | 9 (81.8) | 98.6 | [67] |
| Property (unit) | Testing Method | Catalyst | ASTM D6751 | EN 14214 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CPP | NaOCH3 | /– | KOH | KOH b | NaOH | KOH | Nano-MgO | KOH | ||||
| Density at 25 °C (kg/m3) | [71] | 877 ± 0.040 | - | 877.5 a | 890 a | 875 | - | 869.6 | 880 a | 859.3 | NS | 860–900 |
| Kinematic viscosity at 40 °C (mm2/s) | [71] | 4.95 ± 0.000 | 4.83 | 4.91 | 4.78 | 4.80 | 4.85 | 5.05 | 4.70 | 5.05 | 1.9–6.0 | 3.5–5.0 |
| Acid value (mg KOH/g oil) | [71] | 0.224 ± 0.000 | - | 0.012 | 0.16 | 0.38 | 0.26 | 0.22 | - | - | 0.50 max | 0.50 max |
| Calorific value (MJ/kg) | [72] | 40.70 ± 0.029 | - | - | 38.34 | 45.28 | - | 40.05 | - | 40.06 | NS | NS |
| Cetane number | [73] | 63.05 ± 0.131 | 67.07 | 62.12 | 63 | 67 | - | - | - | 56 | 47 min | 51 min |
| Flash point (°C) | ASTM D 93 | 192 | - | 206 | - | 162 | 135 | 150.5 | 166 | 150.1 | 93 min | 101 min |
| Cloud point (°C) | ASTM D 2500 | +18 | +18 | +10 | +10 | +18 | +18 | +19 | +15 | - | NS | NS |
| Pour point (°C) | ASTM D 97 | +12 | +17 | +3 | +3 | +17 | +17 | +19 | +13 | - | NS | NS |
| Cold filter plugging point (°C) | [74] | +10.6 | - | - | - | +17 | - | +18 | - | +39.70 | NS | NS |
| Reference | This study | [31] | [36] | [32] | [75] | [33] | [34] | [39] | [35] | |||
| Scenario | Reaction Order w.r.t. Individual Reactant | Reaction Kinetics Modeling Equation | Overall Reaction Order, n | R2 | k (min−1) |
|---|---|---|---|---|---|
| 1 | = 0, = 0 | 0 | 0.8583 | 0.09729 | |
| 2 | = 1, = 0 | 1 | 0.9353 | 0.0975 | |
| 3 | = 0, = 1 | 1 | 0.8633 | 0.00419 | |
| 4 | = 1, = 1 | 2 | 0.9441 | 0.00457 | |
| 5 | = 2, = 0 | 2 | 0.9926 | 0.2047 | |
| 6 | = 0, = 2 | 2 | 0.8686 | 1.822 × 10−4 | |
| 7 | = 2, = 1 | 3 | 0.9912 | 0.01027 | |
| 8 | = 1, = 2 | 3 | 0.9523 | 2.161 × 10−4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oladipo, B.; Ojumu, T.V.; Latinwo, L.M.; Betiku, E. Pawpaw (Carica papaya) Peel Waste as a Novel Green Heterogeneous Catalyst for Moringa Oil Methyl Esters Synthesis: Process Optimization and Kinetic Study. Energies 2020, 13, 5834. https://doi.org/10.3390/en13215834
Oladipo B, Ojumu TV, Latinwo LM, Betiku E. Pawpaw (Carica papaya) Peel Waste as a Novel Green Heterogeneous Catalyst for Moringa Oil Methyl Esters Synthesis: Process Optimization and Kinetic Study. Energies. 2020; 13(21):5834. https://doi.org/10.3390/en13215834
Chicago/Turabian StyleOladipo, Babatunde, Tunde V Ojumu, Lekan M Latinwo, and Eriola Betiku. 2020. "Pawpaw (Carica papaya) Peel Waste as a Novel Green Heterogeneous Catalyst for Moringa Oil Methyl Esters Synthesis: Process Optimization and Kinetic Study" Energies 13, no. 21: 5834. https://doi.org/10.3390/en13215834
APA StyleOladipo, B., Ojumu, T. V., Latinwo, L. M., & Betiku, E. (2020). Pawpaw (Carica papaya) Peel Waste as a Novel Green Heterogeneous Catalyst for Moringa Oil Methyl Esters Synthesis: Process Optimization and Kinetic Study. Energies, 13(21), 5834. https://doi.org/10.3390/en13215834