Methanol Electrolysis for Hydrogen Production Using Polymer Electrolyte Membrane: A Mini-Review
Abstract
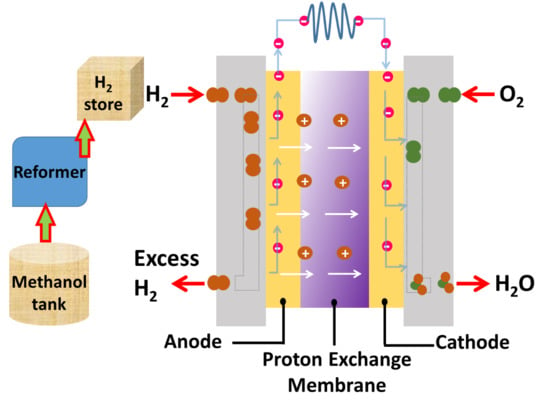
:1. Introduction
Pure Hydrogen Production from Methanol by Electrochemical Method
2. Components of PEM Methanol Electrolyzer
2.1. Bipolar Plates
2.2. Current Collector
- Strong corrosion resistance.
- Good electrical conductivities.
- Good mechanical support to the membrane.
- Effective removal of gases and efficient reaction pathways for fuel to reach catalyst layer.
2.3. Membrane Electrode Assembly
2.3.1. Electrocatalyst for Methanol Electrolysis
2.3.2. Proton Exchange Membrane
3. Optimizing Operating Parameters
4. Methanol Electrolyzer Weight Comparison and Advantages of Methanol Economy
- 185 kg of biomethanol per metric ton of MSW
- 2.8253 × 109 kg × 0.185/0.793 = 659.118 ML
- Overall replacement of motor gasoline by methanol
- 659.118/1143.6 × 100 = 57.63% (by volume)
- (659.118 × 15.8)/(1143.6 × 32.2) × 100 = 28.28% (by energy)
- 1.4865 × 109 kg × 0.185/0.793 = 346.79 ML
- Overall replacement of gasoline by methanol
- 346.79/1143.6 × 100 = 30.32% (By volume)
- (346.79 × 15.8)/(1143.6 × 32.2) = 14.88% (By energy)
5. Future Research Perspectives
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ni, W.; Chen, Z. Synergistic utilization of coal and other energy—Key to low carbon economy. Front. Energy 2011, 5, 1–19. [Google Scholar] [CrossRef]
- Jayakumar, A.; Chalmers, A.; Lie, T.T. A Review of Prospects for Adoption of Fuel Cell Electric Vehicles in New Zealand. IET Electr. Syst. Transp. 2017, 7, 259–266. [Google Scholar] [CrossRef]
- Jayakumar, A. An assessment on polymer electrolyte membrane fuel cell stack components. Appl. Phys. Chem. Multidiscip. Approaches 2018, 3, 23–49. [Google Scholar]
- Cosnier, S.; Gross, A.J.; Le Goff, A.; Holzinger, M. Recent advances on enzymatic glucose/oxygen and hydrogen/oxygen biofuel cells: Achievements and limitations. J. Power Sources 2016, 325, 252–263. [Google Scholar] [CrossRef]
- Ay, M.; Midilli, A.; Dincer, I. Exergetic performance analysis of a PEM fuel cell. Int. J. Energy Res. 2006, 30, 307–321. [Google Scholar] [CrossRef]
- Sasikumar, G.; Muthumeenal, A.; Pethaiah, S.S.; Nachiappan, N.; Balaji, R. Aqueous methanol eletrolysis using proton conducting membrane for hydrogen production. Int. J. Hydrogen Energy 2008, 33, 5905–5910. [Google Scholar] [CrossRef]
- Ghimire, A.; Kumar, G.; Sivagurunathan, P.; Shobana, S.; Saratale, G.D.; Kim, H.W.; Luongo, V.; Esposito, G.; Munoz, R. Bio-hythane production from microalgae biomass: Key challenges and potential opportunities for algal bio-refineries. Bioresour. Technol. 2017, 241, 525–536. [Google Scholar] [CrossRef]
- Gu, W.; Shen, J.P.; Song, C. Hydrogen production from integrated methanol reforming over Cu-ZnO/Al2O3 and Pt/Al2O3 catalysts for PEM fuel cells. Am. Chem. Soc. Div. Fuel Chem. Prepr. 2003, 48, 804. [Google Scholar]
- Susastriawan, A.; Saptoadi, H. Small-scale downdraft gasifiers for biomass gasification: A review. Renew. Sustain. Energy Rev. 2017, 76, 989–1003. [Google Scholar] [CrossRef]
- Ursua, A.; Gandia, L.M.; Sanchis, P. Hydrogen production from water electrolysis: Current status and future trends. Proc. IEEE 2011, 100, 410–426. [Google Scholar] [CrossRef]
- Steele, B.C.; Heinzel, A. Materials for fuel-cell technologies. In Materials for Sustainable Energy: A Collection of Peer-Reviewed Research and Review Articles from Nature Publishing Group; World Scientific: Singapore, 2011; pp. 224–231. [Google Scholar]
- Steele, B.C.; Heinzel, A. Materials for fuel-cell technologies. Nature 2001, 414, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, A.; Sethu, S.P.; Ramos, M.; Robertson, J.; Al-Jumaily, A. A technical review on gas diffusion, mechanism and medium of PEM fuel cell. Ionics 2015, 21, 1–18. [Google Scholar] [CrossRef]
- Sethu, S.P.; Gangadharan, S.; Chan, S.H.; Stimming, U. Development of a novel cost effective methanol electrolyzer stack with Pt-catalyzed membrane. J. Power Sources 2014, 254, 161–167. [Google Scholar] [CrossRef]
- Kumar, J.A.; Kalyani, P.; Saravanan, R. Studies on PEM fuel cells using various alcohols for low power applications. Int. J. Electrochem. Sci. 2008, 3, 961–969. [Google Scholar]
- Atlam, O.; Kolhe, M. Equivalent electrical model for a proton exchange membrane (PEM) electrolyser. Energy Convers. Manag. 2011, 52, 2952–2957. [Google Scholar] [CrossRef]
- Smith, A.; Newborough, M. Low-Cost Polymer Electrolysers and Electrolyser Implementation Scenarios for Carbon Abatement; Rep. Carbon Trust ITM Power: Sheffield, UK, 2006. [Google Scholar]
- Lin, J.; Wu, P.H.; Wycisk, R.; Trivisonno, A.; Pintauro, P.N. Direct methanol fuel cell operation with pre-stretched recast Nafion®. J. Power Sources 2008, 183, 491–497. [Google Scholar] [CrossRef]
- Lin, L.; Zhou, W.; Gao, R.; Yao, S.; Zhang, X.; Xu, W.; Zheng, S.; Jiang, Z.; Yu, Q.; Li, Y.W.; et al. Low-temperature hydrogen production from water and methanol using Pt/α-MoC catalysts. Nature 2017, 544, 80. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, H. Electrocatalysis of Direct Methanol Fuel Cells: From Fundamentals to Applications; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Pethaiah, S.S.; Ulaganathan, M.; Viswanathan, M.R.; Chan, S.H. Fabrication and electrochemical characterization of Pt–Pd impregnated nanocomposite polymer electrolyte membranes for high concentration DMFCs. RSC Adv. 2015, 5, 981–987. [Google Scholar] [CrossRef]
- Fujiwara, N.; Siroma, Z.; Ioroi, T.; Yasuda, K. Rapid evaluation of the electrooxidation of fuel compounds with a multiple-electrode setup for direct polymer electrolyte fuel cells. J. Power Sources 2007, 164, 457–463. [Google Scholar] [CrossRef]
- Narayanan, S.R.; Chun, W.; Jeffries-nakamura, B.; Valdez, T.I. Hydrogen Generation by Electrolysis of Aqueous Organic Solutions. U.S. Patent 6,299,744, 9 October 2001. [Google Scholar]
- Mishra, P.; Krishnan, S.; Rana, S.; Singh, L.; Sakinah, M.; Ab Wahid, Z. Outlook of fermentative hydrogen production techniques: An overview of dark, photo and integrated dark-photo fermentative approach to biomass. Energy Strategy Rev. 2019, 24, 27–37. [Google Scholar] [CrossRef]
- Holton, O.T.; Stevenson, J.W. The role of platinum in proton exchange membrane fuel cells. Platin. Met. Rev. 2013, 57, 259–271. [Google Scholar] [CrossRef]
- Pethaiah, S.S.; Paruthimal Kalaignan, G.; Sasikumar, G.; Ulaganathan, M.; Swaminathan, V. Development of nano-catalyzed membrane for PEM fuel cell applications. J. Solid State Electrochem. 2013, 17, 2917–2925. [Google Scholar] [CrossRef]
- Budak, Y.; Devrim, Y. Comparative study of PV/PEM fuel cell hybrid energy system based on methanol and water electrolysis. Energy Convers. Manag. 2019, 179, 46–57. [Google Scholar] [CrossRef]
- Lonis, F.; Tola, V.; Cau, G. Assessment of integrated energy systems for the production and use of renewable methanol by water electrolysis and CO2 hydrogenation. Fuel 2021, 285, 119160. [Google Scholar] [CrossRef]
- Gurau, V.; Ogunleke, A.; Strickland, F. Design of a methanol reformer for on-board production of hydrogen as fuel for a 3 kW High-Temperature Proton Exchange Membrane Fuel Cell power system. Int. J. Hydrogen Energy 2020, 45, 31745–31759. [Google Scholar] [CrossRef]
- Russell, J.H.; Nuttall, L.J.; Ficket, A. Hydrogen generation by solid polymer electrolyte water electrolysis. Hydrogen Energy 1975, 441–455. [Google Scholar] [CrossRef]
- Ferreira-Aparicio, P.; Chaparro, A.M. Portable Hydrogen Energy Systems; Elsevier Academic Press: Cambridge, MA, USA, 2018; ISBN 978-0-12-813128-2. [Google Scholar]
- Ren, H.; Chae, J. Fuel cells technologies for wireless MEMS. In Wireless MEMS Networks and Applications; Woodhead Publishing: Cambridge, UK, 2017; pp. 35–51. [Google Scholar]
- Dicks, A.L. The role of carbon in fuel cells. J. Power Sources 2006, 156, 128–141. [Google Scholar] [CrossRef]
- Peng, L.; Yi, P.; Lai, X. Design and manufacturing of stainless steel bipolar plates for proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2014, 39, 21127–21153. [Google Scholar] [CrossRef]
- Fu, Y.; Lin, G.; Hou, M.; Wu, B.; Li, H.; Hao, L.; Shao, Z.; Yi, B. Optimized Cr-nitride film on 316L stainless steel as proton exchange membrane fuel cell bipolar plate. Int. J. Hydrogen Energy 2009, 34, 453–458. [Google Scholar] [CrossRef]
- Tang, Y.; Yuan, W.; Pan, M.; Li, Z.; Chen, G.; Li, Y. Experimental investigation of dynamic performance and transient responses of a kW-class PEM fuel cell stack under various load changes. Appl. Energy 2010, 87, 1410–1417. [Google Scholar] [CrossRef]
- Yoon, W.; Huang, X.; Fazzino, P.; Reifsnider, K.L.; Akkaoui, M.A. Evaluation of coated metallic bipolar plates for polymer electrolyte membrane fuel cells. J. Power Sources 2008, 179, 265–273. [Google Scholar] [CrossRef]
- Wang, J.; Sun, J.; Li, S.; Wen, Z.; Ji, S. Surface diffusion modification AISI 304SS stainless steel as bipolar plate material for proton exchange membrane fuel cell. Int. J. Hydrogen Energy 2012, 37, 1140–1144. [Google Scholar] [CrossRef]
- Hermann, A.; Chaudhuri, T.; Spagnol, P. Bipolar plates for PEM fuel cells, a review. Int. J. Hydrogen Energy 2005, 30, 1297–1302. [Google Scholar] [CrossRef]
- Li, X.; Sabir, I. Review of bipolar plates in PEM fuel cells, Flow-field designs. Int. J. Hydrogen Energy 2005, 30, 359–371. [Google Scholar] [CrossRef]
- Antunes, R.A.; Oliveira, M.C.L.; Ett, G.; Ett, V. Corrosion of metal bipolar plates for PEM fuel cells: A review. Int. J. Hydrogen Energy 2010, 35, 3632–3647. [Google Scholar] [CrossRef]
- Pham, A.T.; Baba, T.; Sugiyama, T.; Shudo, T. Efficient hydrogen production from aqueous methanol in a PEM electrolyzer with porous metal flow field, Influence of PTFE treatment of the anode gas diffusion layer. Int. J. Hydrogen Energy 2013, 38, 73–81. [Google Scholar] [CrossRef]
- Tuan, A.; Shudo, T. Efficient hydrogen production from aqueous methanol in a proton exchange membrane electrolyzer with porous metal flow fields. Int. J. Automot. Eng. 2012, 3, 125–130. [Google Scholar] [CrossRef]
- Pham, A.T.; Baba, T.; Shudo, T. Efficient hydrogen production from aqueous methanol in a PEM electrolyzer with porous metal flow field: Influence of change in grain diameter and material of porous metal flow field. Int. J. Hydrog. Energy 2013, 38, 9945–9953. [Google Scholar] [CrossRef]
- Carmo, M.; Fritz, D.L.; Mergel, J.; Stolten, D. A comprehensive review on PEM water electrolysis. Int. J. Hydrogen Energy 2013, 38, 4901–4934. [Google Scholar] [CrossRef]
- Mallick, R.K.; Thombre, S.B.; Shrivastava, N.K. A critical review of the current collector for passive direct methanol fuel cells. J. Power Sources 2015, 285, 510–529. [Google Scholar] [CrossRef]
- Sethu, S.; Viswanathan, M.R.; Mani, U.; Chan, S.H. Evaluation of impregnated nanocomposite membranes for aqueous methanol electrochemical reforming. Solid State Ion. 2015, 283, 16–20. [Google Scholar] [CrossRef]
- Schlapbach, L.; Züttel, A. Hydrogen-storage materials for mobile applications. In Materials for Sustainable Energy: A Collection of Peer-Reviewed Research and Review Articles from Nature Publishing Group; World Scientific: Singapore, 2011; pp. 265–270. [Google Scholar]
- Tang, H.; Wang, S.; Pan, M. A comparative study of CCM and hot-pressed MEAs for PEM fuel cells. J. Power Sources 2007, 170, 140–144. [Google Scholar] [CrossRef]
- Huang, T.; Mao, S.; Zhou, G.; Zhang, Z.; Wen, Z.; Huang, X.; Ci, S.; Chen, J. A high-performance catalyst support for methanol oxidation with graphene and vanadium carbonitride. Nanoscale 2015, 7, 1301–1307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakati, N.; Maiti, J.; Lee, S.H.; Jee, S.H.; Viswanathan, B.; Yoon, Y.S. Anode catalysts for direct methanol fuel cells in acidic media, do we have any alternative for Pt or Pt–Ru? Chem. Rev. 2014, 114, 12397–12429. [Google Scholar] [CrossRef]
- Brookes, C.; Bowker, M.; Wells, P. Catalysts for the selective oxidation of methanol. Catalysts 2016, 6, 92. [Google Scholar] [CrossRef] [Green Version]
- Alia, S.M.; Pylypenko, S.; Neyerlin, K.C.; Kocha, S.S.; Pivovar, B.S. Platinum Nickel Nanowires as Methanol Oxidation Electrocatalysts. J. Electrochem. Soc. 2015, 162, F1299–F1304. [Google Scholar] [CrossRef] [Green Version]
- Garbarino, S.; Ponrouch, A.; Pronovost, S.; Guay, D. Enhanced stability and activity of PtRu nanotubes for methanol electrooxidation. Electrochem. Commun. 2009, 11, 1449–1452. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, X.; Hong, L. Physical and electrochemical characterizations of nanostructured Pd/C and PdNi/C catalysts for methanol oxidation. Electrochem. Commun. 2009, 11, 925–928. [Google Scholar] [CrossRef]
- Liu, H.; Song, C.; Zhang, L.; Zhang, J.; Wang, H.; Wilkinson, D.P. A review of anode catalysis in the direct methanol fuel cell. J. Power Sources 2006, 155, 95–110. [Google Scholar] [CrossRef]
- Liu, J.; Sun, G.; Zhao, F.; Wang, G.; Zhao, G.; Chen, L.; Yi, B.; Xin, Q. Study of sintered stainless steel fiber felt as gas diffusion backing in air-breathing DMFC. J. Power Sources 2004, 133, 175–180. [Google Scholar] [CrossRef]
- Sapountzi, F.M.; Gracia, J.M.; Fredriksson, H.O.; Niemantsverdriet, J.H. Electrocatalysts for the generation of hydrogen, oxygen and synthesis gas. Prog. Energy Combust. Sci. 2017, 58, 1–35. [Google Scholar] [CrossRef] [Green Version]
- Uhm, S.; Lee, J. Accelerated durability test of DMFC electrodes by electrochemical potential cycling. J. Ind. Eng. Chem. 2009, 15, 661–664. [Google Scholar] [CrossRef]
- Guenot, B.; Cretin, M.; Lamy, C. Clean hydrogen generation from the electrocatalytic oxidation of methanol inside a proton exchange membrane electrolysis cell (PEMEC), effect of methanol concentration and working temperature. J. Appl. Electrochem. 2015, 45, 973–981. [Google Scholar] [CrossRef]
- Uhm, S.; Jeon, H.; Kim, T.J.; Lee, J. Clean hydrogen production from methanol–water solutions via power-saved electrolytic reforming process. J. Power Sources 2012, 198, 218–222. [Google Scholar] [CrossRef]
- Wu, M.; Shen, P.K.; Wei, Z.; Song, S.; Nie, M. High activity PtPd-WC/C electrocatalyst for hydrogen evolution reaction. J. Power Sources 2007, 166, 310–316. [Google Scholar] [CrossRef]
- Ju, H.; Giddey, S.; Badwal, S. The role of nanosized SnO2 in Pt-based electrocatalysts for hydrogen production in methanol assisted water electrolysis. Electrochimica Acta 2017, 229, 39–47. [Google Scholar] [CrossRef]
- Hu, Z.; Wu, M.; Wei, Z.; Song, S.; Shen, P.K. Pt-WC/C as a cathode electrocatalyst for hydrogen production by methanol electrolysis. J. Power Sources 2007, 166, 458–461. [Google Scholar] [CrossRef]
- Mahesh, K.N.; Balaji, R.; Dhathathreyan, K.S. Palladium nanoparticles as hydrogen evolution reaction (HER) electrocatalyst in electrochemical methanol reformer. Int. J. Hydrog. Energy 2016, 41, 46–51. [Google Scholar] [CrossRef]
- Chen, L.N.; Hou, K.P.; Liu, Y.S.; Qi, Z.Y.; Zheng, Q.; Lu, Y.H.; Chen, J.Y.; Chen, J.L.; Pao, C.W.; Wang, S.B.; et al. Efficient hydrogen production from methanol using a single-site Pt1/CeO2 catalyst. J. Am. Chem. Soc. 2019, 141, 17995–17999. [Google Scholar] [CrossRef] [Green Version]
- Kuo, C.T.; Lu, Y.; Kovarik, L.; Engelhard, M.; Karim, A.M. Structure sensitivity of acetylene semi-hydrogenation on Pt single atoms and subnanometer clusters. ACS Catal. 2019, 9, 11030–11041. [Google Scholar] [CrossRef]
- Tuomi, S. Hydrogen Evolution Research with non-Noble Metal Catalysts and Methanol Electrolysis. Ph.D. Dissertation, Aalto University, Helsinki, Finland, 2019. [Google Scholar]
- Wu, G. Current challenge and perspective of PGM-free cathode catalysts for PEM fuel cells. Front. Energy 2017, 11, 286–298. [Google Scholar] [CrossRef]
- Xu, G.; Wu, Z.; Wei, Z.; Zhang, W.; Wu, J.; Li, Y.; Li, J.; Qu, K.; Cai, W. Non-destructive fabrication of Nafion/silica composite membrane via swelling-filling modification strategy for high temperature and low humidity PEM fuel cell. Renew. Energy 2020, 153, 935–939. [Google Scholar] [CrossRef]
- Muthumeenal, A.; Pethaiah, S.S.; Nagendran, A. Investigation of SPES as PEM for hydrogen production through electrochemical reforming of aqueous methanol. Renew. Energy 2016, 91, 75–82. [Google Scholar] [CrossRef]
- Meyer, Q.; Zeng, Y.; Zhao, C. In situ and operando characterization of proton exchange membrane fuel cells. Adv. Mater. 2019, 31, 1901900. [Google Scholar] [CrossRef]
- Sun, H.; Sun, G.; Wang, S.; Liu, J.; Zhao, X.; Wang, G.; Xu, H.; Hou, S.; Xin, Q. Pd electroless plated Nafion® membrane for high concentration DMFCs. J. Membr. Sci. 2005, 259, 27–33. [Google Scholar] [CrossRef]
- Hogarth, M.; Hards, G. Direct methanol fuel cells. Platin. Met. Rev. 1996, 40, 150–159. [Google Scholar]
- Palo, D.R.; Dagle, R.A.; Holladay, J.D. Methanol steam reforming for hydrogen production. Chem. Rev. 2007, 107, 3992–4021. [Google Scholar] [CrossRef]
- Take, T.; Tsurutani, K.; Umeda, M. Hydrogen production by methanol–water solution electrolysis. J. Power Sources 2007, 164, 9–16. [Google Scholar] [CrossRef]
- Jayakumar, A.; Ramos, M.; Al-Jumaily, A. A Novel Fuzzy Schema to Control the Temperature and Humidification of PEM Fuel Cell System. In Proceedings of the ASME 2015 13th International Conference on Fuel Cell Science, Engineering and Technology, San Diego, CA, USA, 28 June–2 July 2015. [Google Scholar]
- Sunahiro, S.; Matsui, M.; Takeda, Y.; Yamamoto, O.; Imanishi, N. Rechargeable aqueous lithium–air batteries with an auxiliary electrode for the oxygen evolution. J. Power Sources 2014, 262, 338–343. [Google Scholar] [CrossRef] [Green Version]
- Shamsul, N.; Kamarudin, S.K.; Rahman, N.A.; Kofli, N.T. An overview on the production of bio-methanol as potential renewable energy. Renew. Sustain. Energy Rev. 2014, 33, 578–588. [Google Scholar] [CrossRef]
- Abdalla, A.M.; Hossain, S.; Petra, M.; Ghasemi, M.; Azad, A.K. Achievements and trends of solid oxide fuel cells in clean energy field: A perspective review. Front. Energy 2018, 14, 359–382. [Google Scholar] [CrossRef]
- Zohuri, B. Large-Scale Hydrogen Production. Hydrog. Energy 2019, 229–255. [Google Scholar] [CrossRef]











| Fuel | Electrical Potential for Electrolysis (V) | Energy Density (kWh/kg) |
|---|---|---|
| Water | 1.23 V | -- |
| Methanol | 0.03 V | 6.1 |
| Ethanol | 1.145 V | 8.1 |
| Ammonia | -- | 5.7 |
| Anode Catalyst | Cathode Catalyst | Membrane | Energy Consumption kWh (Nm3)−1 | Reference |
|---|---|---|---|---|
| 40% Pt–Ru/C | 20% Pt/C | Nafion-117 | 1.48–2.87 | [23] |
| Pt–Ru (1:1)/C | Pt/C | Nafion-117 | 1–1.2 | [60] |
| Pt–Ru (1:1) black | Pt black | Nafion-115 | 1.46 | [61] |
| 50% Pt–Ru/C | 50% Pt/C | Nafion-117 | 1.38–1.78 | [43] |
| Hydrogen Production Method | Weight of 1 Nm3/h Hydrogen Generators (kg) | Hydrogen Purity (%) | Company | References |
|---|---|---|---|---|
| Methanol electrolyzer | 65 | 99 | Home made | [14] |
| Water electrolyzer | 250 | 99.99% | Hydrogenics | |
| Methanol steam reformer | 58 | 99.95% | Element 1 (S-Series) | |
| Methanol steam reformer | 25 | 75% | WS FLOX (FPMC1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pethaiah, S.S.; Sadasivuni, K.K.; Jayakumar, A.; Ponnamma, D.; Tiwary, C.S.; Sasikumar, G. Methanol Electrolysis for Hydrogen Production Using Polymer Electrolyte Membrane: A Mini-Review. Energies 2020, 13, 5879. https://doi.org/10.3390/en13225879
Pethaiah SS, Sadasivuni KK, Jayakumar A, Ponnamma D, Tiwary CS, Sasikumar G. Methanol Electrolysis for Hydrogen Production Using Polymer Electrolyte Membrane: A Mini-Review. Energies. 2020; 13(22):5879. https://doi.org/10.3390/en13225879
Chicago/Turabian StylePethaiah, Sethu Sundar, Kishor Kumar Sadasivuni, Arunkumar Jayakumar, Deepalekshmi Ponnamma, Chandra Sekhar Tiwary, and Gangadharan Sasikumar. 2020. "Methanol Electrolysis for Hydrogen Production Using Polymer Electrolyte Membrane: A Mini-Review" Energies 13, no. 22: 5879. https://doi.org/10.3390/en13225879
APA StylePethaiah, S. S., Sadasivuni, K. K., Jayakumar, A., Ponnamma, D., Tiwary, C. S., & Sasikumar, G. (2020). Methanol Electrolysis for Hydrogen Production Using Polymer Electrolyte Membrane: A Mini-Review. Energies, 13(22), 5879. https://doi.org/10.3390/en13225879