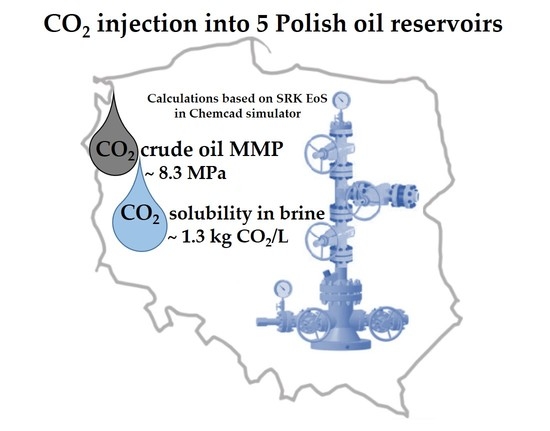
Chemistry of Reservoir Fluids in the Aspect of CO2 Injection for Selected Oil Reservoirs in Poland
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reservoir Fluids
2.2. Analytical Methods
2.3. Phase Equilibrium Calculations
2.3.1. CO2–Oil MMP Calculations
2.3.2. Modeling of CO2 and CH4 Solubility in Brines
3. Results and Discussions
3.1. Crude Oils Characterization
3.2. CO2–Oil MMP Calculations
3.3. CO2 and CH4 Solubility in Brines
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A









References
- General Secretariat of the Council. Conclusions on 2030 Climate and Energy Policy Framework. Note, European Commission (EC), Brussels; October 2014. Available online: http://ec.europa.eu/clima/policies/2030/documentation_en.%0Ahtm (accessed on 22 October 2019).
- Communication from the Commission to the European Parliament, the European Council, the Council, the European Econnomic and Social Committee and the Committee of the Regions. The European Green Deal. COM/2019/640 Final. Available online: https://eur-lex.europa.eu/resource.html?uri=cellar:b828d165-1c22-11ea-8c1f-01aa75ed71a1.0002.02/DOC_1&format=PDF (accessed on 22 September 2020).
- Wang, M.; Joel, A.S.; Ramshaw, C.; Eimer, D.; Musa, N.M. Process intensification for post-combustion CO2 capture with chemical absorption: A critical review. Appl. Energy 2015, 158, 275–291. [Google Scholar] [CrossRef]
- Czarnota, R.; Knapik, E.; Wojnarowski, P.; Janiga, D.; Stopa, J. Carbon dioxide separation technologies. Arch. Min. Sci. 2019, 64, 487–498. [Google Scholar]
- Wang, M.; Lawal, A.; Stephenson, P.; Sidders, J.; Ramshaw, C. Post-combustion CO2 capture with chemical absorption: A state-of-the-art review. Chem. Eng. Res. Des. 2011, 89, 1609–1624. [Google Scholar] [CrossRef] [Green Version]
- Yildirim, O.; Kiss, A.A.; Hüser, N.; Leßmann, K.; Kenig, E.Y. Reactive absorption in chemical process industry: A review on current activities. Chem. Eng. J. 2012, 213, 371–391. [Google Scholar] [CrossRef]
- Karayannis, V.; Charalampides, G.; Lakioti, E. Socio-economic aspects of CCS technologies. Procedia Econ. Financ. 2014, 14, 295–302. [Google Scholar] [CrossRef] [Green Version]
- Venkatraman, A.; Lake, L.W.; Johns, R.T. Modelling the impact of geochemical reactions on hydrocarbon phase behavior during CO2 gas injection for enhanced oil recovery. Fluid Phase Equilib. 2015, 402, 56–68. [Google Scholar] [CrossRef] [Green Version]
- Yadala, S.; Cremaschi, S. Design and optimization of artificial cultivation units for algae production. Energy 2014, 78, 23–39. [Google Scholar] [CrossRef]
- Zhang, C.; Jun, K.-W.; Kwak, G.; Lee, Y.-J.; Park, H.-G. Efficient utilization of carbon dioxide in a gas-to-methanol process composed of CO2/steam–mixed reforming and methanol synthesis. J. CO2 Util. 2016, 16, 1–7. [Google Scholar] [CrossRef]
- Ackiewicz, M.; Foster, C.; Bonijoly, D.; Ramsak, P.; Al-Eidan, A.; Surridge, T. CO2 Utilisation Options—Phase 1 Report; Technical group report; Carbon Sequestration Leadership Forum (CSLF): Washington, DC, USA, 2012. [Google Scholar]
- Kurz, B.A.; Sorensen, J.A.; Hawthorne, S.B.; Smith, S.A.; Sanei, H.; Ordakani, O.; Walls, J.; Jin, L.; Butler, S.K.; Beddoe, J.C.; et al. The influence of organics on supercritical CO2 migration in organic- rich shales. In Proceedings of the Unconventional Resources Technology Conference, Houston, TX, USA, 23–25 July 2018. [Google Scholar]
- Gamadi, T.D.; Sheng, J.J.; Soliman, M.Y.; Menouar, H.; Watson, M.C.; Emadibaladehi, H. An experimental study of cyclic CO2 injection to improve shale oil recovery. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 12–16 April 2014. [Google Scholar]
- Song, C.; Yang, D. Experimental and numerical evaluation of CO2 huff-n-puff processes in Bakken formation. Fuel 2017, 190, 145–162. [Google Scholar] [CrossRef]
- Du, F.; Nojabaei, B. A review of gas injection in shale reservoirs: Enhanced oil/gas recovery approaches and greenhouse gas control. Energies 2019, 12, 2355. [Google Scholar] [CrossRef] [Green Version]
- Jia, B.; Tsau, J.-S.; Barati, R. A review of the current progress of CO2 injection EOR and carbon storage in shale oil reservoirs. Fuel 2019, 236, 404–427. [Google Scholar] [CrossRef]
- Uliasz-Misiak, B.; Przybycin, A. The perspectives and barriers for the implementation of CCS in Poland. Greenh. Gases Sci. Technol. 2016, 6, 7–18. [Google Scholar] [CrossRef]
- Institute of the Environmental Protection-National Research Institute. The National Centre for Emissions Management. Krajowy Raport Inwentaryzacyjny 2019. Inwentaryzacja Gazów Cieplarnianych dla lat 1988–2017; Institute of the Environmental Protection-National Research Institute: Warsaw, Poland, 2019.
- Gutmann, K.; Huscher, J.; Urbaniak, D.; White, A.; Schaible, C.; Bricke, M. Europe’s Dirty 30 How the EU’s Coal-Fired Power Plants Are Undermining Its Climate Efforts Highlights. 2014. Available online: http://awsassets.panda.org/downloads/dirty_30_report_finale.pdf (accessed on 12 February 2016).
- Więcław-Solny, L.; Tatarczuk, A.; Stec, M.; Krótki, A. Advanced CO2 capture pilot plant at tauron’s coal-fired power plant: Initial results and further opportunities. Energy Procedia 2014, 63, 6318–6322. [Google Scholar] [CrossRef] [Green Version]
- Stec, M.; Tatarczuk, A.; Wiecław-Solny, L.; Krótki, A.; Spietz, T.; Wilk, A.; Śpiewak, D. Demonstration of a post-combustion carbon capture pilot plant using amine-based solvents at the Łaziska Power Plant in Poland. Clean Technol. Environ. Policy 2016, 18, 151–160. [Google Scholar] [CrossRef] [Green Version]
- Wolf, K.H.A.A.; van Bergen, F.; Ephraim, R.; Pagnier, H. Determination of the cleat angle distribution of the RECOPOL coal seams, using CT-scans and image analysis on drilling cuttings and coal blocks. Int. J. Coal Geol. 2008, 73, 259–272. [Google Scholar] [CrossRef]
- Lubaś, J. Pionierskie doswiadczenia Polski w zakresie sekwestracji dwutlenku wegla. Prz. Geol. 2007, 55, 663–665. [Google Scholar]
- Kern, F.; Gaede, J.; Meadowcroft, J.; Watson, J. The political economy of carbon capture and storage: An analysis of two demonstration projects. Technol. Forecast. Soc. Chang. 2016, 102, 250–260. [Google Scholar] [CrossRef]
- Eccles, J.K.; Pratson, L. A “carbonshed” assessment of small-vs. large-scale CCS deployment in the continental US. Appl. Energy 2014, 113, 352–361. [Google Scholar] [CrossRef]
- Tarkowski, R.; Misiak, B.U.; Wójcicki, A. CO2 storage capacity of deep aquifers and hydrocarbon fields in Poland-EU GeoCapacity. Project results. Energy Procedia 2009, 1, 2671–2677. [Google Scholar]
- Wojnarowski, P. Analiza mozliwosci zwiekszenia efektywnosci wydobycia ropy naftowej z Polskich złóż w oparciu metody EOR. Miner. Resour. Manag. 2012, 28, 47–58. [Google Scholar]
- Mikołajczak, E.; Kosowski, P.; Stopa, J.; Wartak, J. Analysis and selection of CO2 sources for CCS-EOR projects in oil fields clusters in Poland. AGH Drill. Oil Gas 2018, 35, 295–306. [Google Scholar] [CrossRef]
- Kharaka, Y.K.; Maest, A.S.; Carothers, W.W.; Law, L.M.; Lamothe, P.J.; Fries, T.L. Geochemistry of metal-rich brines from central Mississippi Salt Dome basin, USA. Appl. Geochem. 1987, 2, 543–561. [Google Scholar] [CrossRef]
- Eaton, A. (Ed.) Standard Methods for the Examination of Water and Wastewater, 21st ed.; APHA-AWWA-WEF: Washington, DC, USA, 2005. [Google Scholar]
- Gonzalez, A.; Coquelet, C.; Paricaud, P.; Chapoy, A. Comparative study of vapour-liquid equilibrium and density modelling of mixtures related to carbon capture and storage with the SRK, PR, PC-SAFT and SAFT-VR Mie equations of state for industrial uses. Fluid Phase Equilib. 2017, 440, 19–35. [Google Scholar]
- Valderrama, J.O.; Silva, A. Modified Soave-Redlich-Kwong equations of state applied to mixtures containing supercritical carbon dioxide. Korean J. Chem. Eng. 2003, 20, 709–715. [Google Scholar] [CrossRef]
- Karim, A.M.A.; Abdel-Rahman, Z.A.; Hadi, A. Solubility prediction of CO2 in several physical liquid solvents using Chemcad and Hysys simulators. Dilaya J. Eng. Sci. 2010, 3, 356–373. [Google Scholar]
- Akberov, R.R. Calculating the vapor—Liquid phase equilibrium for multicomponent systems using the Soave-Redlich-Kwong equation. Theor. Found. Chem. Eng. 2011, 45, 312–318. [Google Scholar] [CrossRef]
- Choubineh, A.; Helalizadeh, A.; Wood, D.A. The impacts of gas impurities on the minimum miscibility pressure of injected CO2-rich gas—Crude oil systems and enhanced oil recovery potential. Pet. Sci. 2019, 16, 117–126. [Google Scholar] [CrossRef] [Green Version]
- Bethke, C.M. Geochemical Reaction Modeling: Concepts and Applications; Oxford University Press: Oxford, UK, 1996; pp. 110–150. [Google Scholar]
- Perez-Villasenor, F.; Iglesias-Silva, G.A. Osmotic and activity coefficients using a modified Pitzer equation for strong electrolytes 1:1 and 1:2 at 298.15 K. Ind. Eng. Chem. Res. 2002, 41, 1031–1037. [Google Scholar] [CrossRef]
- Ayirala, S.C.; Xu, W.; Rao, D.N. Interfacial behaviour of complex hydrocarbon fluids at elevated pressures andtemperatures. Can. J. Chem. Eng. 2006, 84, 22–32. [Google Scholar] [CrossRef]
- Asghari, K.; Torabi, F. Effect of miscible and immiscible CO2 injection on gravity drainage: Experimental and simulation results. In Proceedings of the SPE Symposium on Improved Oil Recovery, Tulsa, OK, USA, 20–23 April 2008. [Google Scholar]
- Song, Y.-C.; Zhu, N.J.; Yu, L.; Zhao, J.-F.; Liu, W.-G.; Zhang, Y.; Zhao, Y.-C.; Jiang, L.-L. Magnetic resonance imaging study on the miscibility of a CO2/n-decane. Chin. Phys. Lett. 2011, 28, 096401. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, L.; Tang, L.; Song, Y.; Zhao, J.; Zhang, Y.; Wang, D.; Yang, M. Minimum miscibility pressure estimation for a CO2/n-decane system in porous media by X-ray CT. Exp. Fluids 2015, 56, 7. [Google Scholar] [CrossRef]
- Elsharkawy, A.; Poettmann, F.; Christiansen, R. Measuring CO2 minimum miscibility pressures: Slim-tube or rising-bubble method? Energy Fuels 1996, 10, 443–449. [Google Scholar] [CrossRef]
- Czarnota, R.; Janiga, D.; Stopa, J.; Wojnarowski, P. Determination of minimum miscibility pressure for CO2 and oil system using acoustically monitored separator. J. CO2 Util. 2017, 17, 32–36. [Google Scholar] [CrossRef]
- Zhang, P.Y.; Huang, S.; Sayegh, S.; Zhou, X.L. Effect of CO2 impurities on gas-injection EOR processes. In Proceedings of the SPE/DOE Symposium on Improved Oil Recovery, Tulsa, OK, USA, 17–21 April 2004. [Google Scholar]
- Karnkowski, P. Oil and Gas Deposits in Poland; Geosynoptics Society GEOS: Cracow, Poland, 1999. [Google Scholar]
- Dong, M.; Huang, S.; Srivastava, R. Effect of solution gas in oil on CO2 minimum miscibility pressure. J. Can. Pet. Technol. 2000, 11, 53–61. [Google Scholar]
- Alston, R.B.; Kokolis, G.P.; James, C.F. CO2 minimum miscibility pressure: A correlation for impure CO2 streams and live oil systems. Soc. Pet. Eng. J. 1985, 25, 268–274. [Google Scholar] [CrossRef]
- Jia, B.; Tsau, J.-S.; Barati, R. Role of molecular diffusion in heterogeneous, naturally fractured shale reservoirs during CO2 huff-n-puff. J. Pet. Sci. Eng. 2018, 164, 31–42. [Google Scholar] [CrossRef]
- Jia, B.; Tsau, J.-S.; Barati, R. Measurement of CO2 diffusion coefficient in the oil-saturated porous media. J. Pet. Sci. Eng. 2019, 181, 106189. [Google Scholar] [CrossRef]
- Zhou, X.; Jiang, Q.; Yuan, Q.; Zhang, L.; Feng, J.; Chu, B.; Zeng, F.; Zhu, G. Determining CO2 diffusion coefficient in heavy oil in bulk phase and in porous media using experimental and mathematical modeling methods. Fuel 2020, 263, 116205. [Google Scholar] [CrossRef]
- Wang, S.; Ma, M.; Chen, S. Application of PC-SAFT equation of state for CO2 minimum miscibility pressure prediction in nanopores. In Proceedings of the SPE Improved Oil Recovery Conference, Tulsa, OK, USA, 11–13 April 2006. [Google Scholar]
- McLing, T.; Smith, W.; Smith, R. Utilizing rare earth elements as tracers in high TDS reservoir brines in CCS applications. Energy Procedia 2014, 63, 3963–3974. [Google Scholar] [CrossRef] [Green Version]
- Rohmer, J.; Pluymakers, A.; Renard, F. Mechano-chemical interactions in sedimentary rocks in the context of CO2 storage: Weak acid, weak effects? Earth Sci. Rev. 2016, 157, 86–110. [Google Scholar] [CrossRef]
- Kim, S.; Santamarina, J.C. Geometry-coupled reactive fluid transport at the fracture scale: Application to CO2 geologic storage. Geofluids 2016, 16, 329–341. [Google Scholar] [CrossRef] [Green Version]
- Gaus, I. Role and impact of CO2-rock interactions during CO2 storage in sedimentary rocks. Int. J. Greenh. Gas Control 2010, 4, 73–89. [Google Scholar] [CrossRef]
- Adamczyk, K.; Schwarz-Premont, M.; Pines, D.; Pines, E.; Nibbering, E. Real-time observation of carbonic acid formation in aqueous solution. Science 2009, 326, 1690–1694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaus, I.; Audigane, P.; Andre, L.; Lions, J.; Jacquemet, N.; Durst, P.; Czernichowski-Lauriol, I.; Azaroual, M. Geochemical and solute transport modelling for CO2 storage, what to expect from it? Int. J. Greenh. Gas Control 2008, 2, 605–625. [Google Scholar] [CrossRef] [Green Version]
- Rimmelé, G.; Barlet-Gouédard, V.; Renard, F. Evolution of the petrophysical and mineralogical properties of two reservoir rocks under thermodynamic conditions relevant for CO2 geological storage at 3 km depth. Oil Gas Sci. Technol. 2010, 65, 565–580. [Google Scholar] [CrossRef] [Green Version]
- Hangx, S.J.T.; Pluymakers, A.M.H.; Ten Hove, A.; Spiers, C.J. The effects of lateral variations in rock composition and texture on anhydrite caprock integrity of CO2 storage systems. Int. J. Rock Mech. Min. Sci. 2014, 69, 80–92. [Google Scholar] [CrossRef]
- Gilbert, K.; Bennett, P.C.; Wolfe, W.; Zhang, T.; Romanak, K.D. CO2 solubility in aqueous solutions containing Na+, Ca2+, Cl−, SO42− and HCO3−: The effects of electrostricted water and ion hydration thermodynamics. Appl. Geochem. 2016, 67, 59–67. [Google Scholar] [CrossRef] [Green Version]






| Parameter/Field | Method | PL1 | SP1 | SP2 | LP1 | LP2 |
|---|---|---|---|---|---|---|
| Density (g/L) | ASTM D287 | 795 | 843 | 837 | 837 | 827 |
| Viscosity at 313 K (mm2/s) | ASTM D445 | 1.95 | 5.04 | 4.01 | 4.32 | 2.01 |
| Acid number (mg KOH/g) | ASTM D974 | 0.356 | 0.172 | 0.198 | 0.427 | 0.246 |
| Asphaltene content (wt. %) | ASTM D6560 | 0.953 | 0.749 | 1.035 | 1.819 | 0.997 |
| Conradson carbon residue (wt. %) | ASTM D189 | 0.544 | 2.566 | 2.195 | 0.901 | 0.533 |
| Component/Field | PL1 | SP1 | SP2 | LP1 | LP2 |
|---|---|---|---|---|---|
| C6 | 7.63 | 4.51 | 6.22 | 3.75 | 4.23 |
| C7 | 9.88 | 5.43 | 7.53 | 4.4 | 6.98 |
| C8 | 11.44 | 5.06 | 7.56 | 6.31 | 8.53 |
| C9 | 10.15 | 5.88 | 7.36 | 7.30 | 9.32 |
| C10 | 7.12 | 4.32 | 5.30 | 6.73 | 9.35 |
| C11 | 5.80 | 3.72 | 4.47 | 7.18 | 8.19 |
| C12 | 6.14 | 5.15 | 6.11 | 7.57 | 8.02 |
| C13 | 5.06 | 4.97 | 5.50 | 6.70 | 7.25 |
| C14 | 5.31 | 5.27 | 5.48 | 7.42 | 6.74 |
| C15 | 3.93 | 4.30 | 4.26 | 6.32 | 5.37 |
| C16 | 3.36 | 4.53 | 4.36 | 4.93 | 3.54 |
| C17 | 3.31 | 5.45 | 4.99 | 4.97 | 3.54 |
| C18 | 3.55 | 3.91 | 3.41 | 3.39 | 2.35 |
| C19 | 2.48 | 4.00 | 2.81 | 3.37 | 2.15 |
| C20 | 1.93 | 4.08 | 3.03 | 2.84 | 1.57 |
| C21 | 1.34 | 3.39 | 3.24 | 2.28 | 1.52 |
| C22 | 1.50 | 3.23 | 2.68 | 2.31 | 1.59 |
| C23 | 1.31 | 3.02 | 2.56 | 2.33 | 1.30 |
| C24 | 1.34 | 2.86 | 2.40 | 1.94 | 1.12 |
| C25 | 7.42 | 16.92 | 10.73 | 7.96 | 7.34 |
| Parameter/Field | PL1 | SP1 | SP2 | LP1 | LP2 |
|---|---|---|---|---|---|
| Initial reservoir pressure (MPa) | 55.65 | 24.4 | 21.15 | 6.40 | 8.84 |
| Reservoir temperature (K) | 392 | 338 | 325 | 299 | 307 |
| Oil recovery factor, Ro (-) | 0.088 | 0.471 | 0.204 | 0.347 | 0.544 |
| Calculated MMP at 313 K (MPa) | 8.28 | 8.45 | 8.33 | 8.38 | 8.25 |
| Calculated MMP at reservoir temperature (MPa) | 18.10 | 12.93 | 10.55 | 6.29 | 7.38 |
| Parameter | PL1 | LP1 | LP2 | SP1 | SP2 |
|---|---|---|---|---|---|
| pH | 6.2 | 7.6 | 8.6 | 8.0 | 8.9 |
| Conductivity (mS/cm) | 1080 | 127 | 52.5 | 31 | 29 |
| Total suspended solids (g/L) | 0.056 | 0.188 | 0.033 | 0.363 | 0.215 |
| Total dissolved solids (g/L) | 378.900 | 50.105 | 17.515 | 20.780 | 19.580 |
| Cl− (g/L) | 174.410 | 26.394 | 8.644 | 10.630 | 9.794 |
| SO4 2− (g/L) | 3.980 | 2.405 | 1.550 | 0.316 | 0.322 |
| CO3 2− (g/L) | 0.000 | 0.000 | 0.000 | 0.002 | 0.350 |
| HCO3− (g/L) | 0.000 | 0.000 | 0.000 | 0.390 | 2.385 |
| Na+ (g/L) | 75.200 | 13.820 | 5.304 | 7.379 | 7.866 |
| K+ (g/L) | 4.527 | 0.483 | 0.148 | 0.048 | 0.063 |
| Ca2+ (g/L) | 29.852 | 1.403 | 0.532 | 0.032 | 0.060 |
| Mg2+ (g/L) | 2.553 | 1.386 | 0.255 | 0.021 | 0.013 |
| Reservoir | PL1 | LP1 | LP2 | SP1 | SP2 |
|---|---|---|---|---|---|
| CO2 solubility (mol/kg brine) | 1.792 | 1.283 | 1.219 | 1.366 | 1.365 |
| CH4 solubility (mol/kg brine) | 0.421 | 0.083 | 0.102 | 0.208 | 0.198 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knapik, E.; Chruszcz-Lipska, K. Chemistry of Reservoir Fluids in the Aspect of CO2 Injection for Selected Oil Reservoirs in Poland. Energies 2020, 13, 6456. https://doi.org/10.3390/en13236456
Knapik E, Chruszcz-Lipska K. Chemistry of Reservoir Fluids in the Aspect of CO2 Injection for Selected Oil Reservoirs in Poland. Energies. 2020; 13(23):6456. https://doi.org/10.3390/en13236456
Chicago/Turabian StyleKnapik, Ewa, and Katarzyna Chruszcz-Lipska. 2020. "Chemistry of Reservoir Fluids in the Aspect of CO2 Injection for Selected Oil Reservoirs in Poland" Energies 13, no. 23: 6456. https://doi.org/10.3390/en13236456
APA StyleKnapik, E., & Chruszcz-Lipska, K. (2020). Chemistry of Reservoir Fluids in the Aspect of CO2 Injection for Selected Oil Reservoirs in Poland. Energies, 13(23), 6456. https://doi.org/10.3390/en13236456