Solubility and Diffusivity of Polar and Non-Polar Molecules in Polyethylene-Aluminum Oxide Nanocomposites for HVDC Applications
Abstract
:1. Introduction
2. Material and Methods
2.1. Material Preparation (LDPE and LDPE/Al2O3 Nanocomposites)
2.2. Material Characterization
2.3. Solubility (Sorption-Desorption)
2.4. Diffusivity (Sorption/Desorption)
3. Results and Discussion
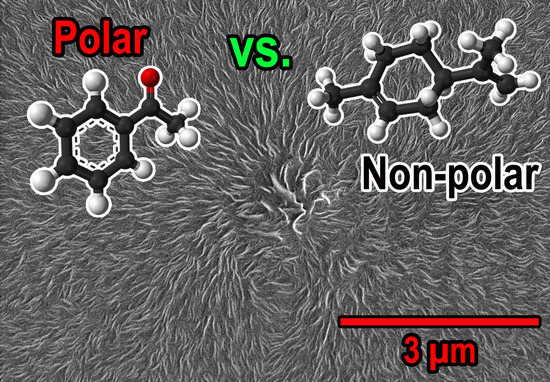
3.1. Material Characterization (SEM, Porosity and DSC)
3.2. Solubility
3.3. Diffusion
3.4. Influence of Particle Size on the Solubility
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Montanari, G.C.; Laurent, C.; Teyssedre, G.; Campus, A.; Nilsson, U.H. From LDPE to XLPE: Investigating the change of electrical properties. Part I: Space charge, conduction and lifetime. IEEE Trans. Dielectr. Electr. Insul. 2005, 12, 438–446. [Google Scholar] [CrossRef]
- Hanley, T.L.; Burford, R.P.; Fleming, R.J.; Barber, K.W. A general review of polymeric insulation for use in HVDC cables. IEEE Electr. Insul. Mag. 2003, 19, 13–24. [Google Scholar] [CrossRef]
- Fothergill, J.C. The Coming of Age of HVDC Extruded Power Cables. Electr. Insul. Conf. (EIC) 2014, 124–137. [Google Scholar] [CrossRef]
- Andersson, M.; Hynynen, J.; Andersson, M.; Englund, V.; Hagstrand, P.A.; Gkourmpis, T.; Müller, C. Highly insulating polyethylene blends for HVDC power cables. ACS Macro Lett. 2017, 6, 78. [Google Scholar] [CrossRef]
- Pallon, L.; Hoang, A.; Pourrahimi, A.; Hedenqvist, M.; Nilsson, F.; Gubanski, S.; Gedde, U.W.; Olsson, R.T. The impact of MgO nanoparticle interface in ultra-insulating polyethylene nanocomposites for high voltage DC cables. J. Mater. Chem. 2016, 4, 8590–8601. [Google Scholar] [CrossRef]
- Pourrahimi, A.M.; Hoang, T.A.; Liu, D.; Pallon, L.K.; Gubanski, S.; Olsson, R.T.; Gedde, U.W.; Hedenqvist, M.S. Highly Efficient Interfaces in Nanocomposites Based on Polyethylene and ZnO Nano/Hierarchical Particles: A Novel Approach toward Ultralow Electrical Conductivity Insulations. Adv. Mater. 2016, 28, 8651–8657. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Hoang, A.; Pourahimi, A.; Pallon, L.K.; Nilsson, F.; Gubanski, S.; Olsson, R.T.; Hedenqvist, M.S.; Gedde, U.W. Influence of nanoparticle surface coating on electrical conductivity of polyethylene/aluminium oxide nanocomposites for HVDC cable insulations. IEEE Trans. Dielectr. Electr. Insul. 2017, 24. [Google Scholar] [CrossRef]
- Nilsson, F.; Karlsson, M.; Pallon, L.; Giacinti, M.; Olsson, R.T.; Venturi, D.; Gedde, U.W.; Hedeqvist, M.S. Influence of water update on the electrical DC-conductivity of insulating LDPE/MgO nanocomposites. Compos. Sci. Technol. 2017, 152, 11–19. [Google Scholar] [CrossRef]
- Pourrahimi, A.M.; Olsson, R.T.; Hedenqvist, M.S. The Role of Interfaces in Polyethylene/Metal-Oxide Nanocomposites for Ultrahigh-Voltage Insulating Materials. Adv. Mater. 2017, 30. [Google Scholar] [CrossRef]
- Lewis, T. Charge Transport in Polyethylene Nano Dielectrics. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 497–502. [Google Scholar] [CrossRef]
- Nelson, J. Dielectric Polymer Nanocomposites; Springer: New York, NY, USA, 2014. [Google Scholar]
- Gao, Y.; Xu, B.; Wang, X.; Jia, T. Charge transport in low density polyethylene based micro/nano-composite with improved thermal conductivity. J. Phys. D Appl. Phys. 2019, 52, 285–302. [Google Scholar] [CrossRef]
- Tian, F.; Lei, Q.; Wang, X.; Wang, Y. Effect of deep trapping states on space charge suppression in polyethylene/ZnO nanocomposite. Appl. Phys. Lett. 2011, 99, 142903. [Google Scholar] [CrossRef]
- Liu, D.; Pourrahimi, A.; Pallon, L.; Sánchez, C.C.; Olsson, R.T.; Hedenqvist, M.S.; Fogelström, L.; Malmström, E.; Gedde, U.W. Interactions between a phenolic antioxidant, moisture, peroxide and crosslinking by-products with metal oxide nanoparticles in branched polyethylene. Polym. Degrad. Stab. 2016, 125, 21–32. [Google Scholar] [CrossRef]
- Wang, S.J.; Zha, J.W.; Li, W.K.; Dang, Z.M. Distinctive electrical properties in sandwich structured Al2O3/LDPE nanocomposites. Appl. Phys. Lett. 2016, 108, 092902. [Google Scholar] [CrossRef]
- Wang, S.J.; Zha, J.W.; Wu, Y.H.; Ren, L.; Dang, Z.M.; Wu, J.I. Preparation, microstructure and properties of polyethylene/aluminium nanocomposites for HVDC insulation. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 3350–3356. [Google Scholar] [CrossRef]
- Zha, J.W.; Yan, H.D.; Li, W.K.; Zhang, D.L.; Dang, Z.M. Sandwiched structure effect on space charge chargecteristics of aluminium/polyethylene nanocompomposites. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 1365–1371. [Google Scholar]
- Zhou, Y.; Hu, J.; Dang, B.; He, J. Effect of different nanoparticles on tuning electrical properties of polypropylene nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 1380–1389. [Google Scholar] [CrossRef]
- Ciuprina, F.; Zaharescu, T.; Plesa, I. Effects of y-radiation on dielectric properties of LDPE-Al2O3 nanocomposites. Radiat. Phys. Chem. 2013, 84, 145–150. [Google Scholar] [CrossRef]
- Elimat, Z.M.; Zihlif, A.M.; Schulte, K.I.; de la Vega, A.; Gagosta, G. Electrical Characterization of polyethylene oxide alumina composite. J. Thermoplast. Compos. Mater. 2011, 26, 176–192. [Google Scholar] [CrossRef]
- Panaitescu, D.; Ciuprina, F.; Iorga, M.; Frone, A.; Radovici, C.; Ghiurea, M.; Sever, S.; Plesa, I. Effects of SiO2 and Al2O3 nanofillers on polyethylene properties. J. Appl. Polym. Sci. 2011, 122, 1921–1935. [Google Scholar] [CrossRef]
- Jose, J.P.; Thomas, S. Aluminia-clay nanoscale hybrid filler assembling in cross-linked polyethylene based nanocomposites: Mechanics and thermal properties. Phys. Chem. Chemphys. 2014, 16, 14730. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Cao, X.Y.; Ma, Y.M.; Ke, Y.C.; Zhang, J.K.; Wang, F.S. The effects of particle size and content on the thermal conductivity and mechanical properties of Al2O3/high density polyethylene (HDPE) composites. Polym. Lett. 2011, 5, 581–590. [Google Scholar] [CrossRef]
- Park, Y.J.; Kwon, J.H.; Sin, J.Y.; Hwang, J.N.; Seo, C.W.; Kim, J.H.; Lim, K.J. DC conductivity and breakdown characteristics of Al2O3/crosslinked polyethylene nanocomposites for high voltage direct current transmission cable insulation. Jpn. J. Appl. Phys. 2014, 53, 08NL05. [Google Scholar] [CrossRef]
- Tanaka, T.; Imai, T. Advances in nanodielectric materials over the past 50 years. IEEE Electr. Insul. Mag. 2013, 12, 10–23. [Google Scholar] [CrossRef]
- Reed, C. Advances in polymer dielectrics over the past 50 years. IEEE Electr. Insul. Mag. 2013, 12, 58–62. [Google Scholar] [CrossRef]
- Teyssedre, G.; Laurent, C. Advances in high-field insulating polymeric materials over the past 50 years. IEEE Electr. Insul. Mag. 2013, 12, 26–36. [Google Scholar] [CrossRef]
- Zhou, Y.; Peng, S.; Hu, J.; He, J. Polymeric insulation materials for HVDC cables: Development, challenges and future prespective. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 1308–1381. [Google Scholar] [CrossRef]
- Plesa, I.; Nothingher, P.V.; Schlögl, S.; Sumereder, C.; Muhr, M. Review: Properties of polymer composites used in high voltage applications. Polymers 2016, 8, 173. [Google Scholar] [CrossRef]
- Plesa, I.; Nothingher, P.V.; Stancu, C.; Wiesbrock, F.; Schlögl, S. Review: Polyethylene nanocomposites for power cable insulations. Polymers 2019, 11, 24. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Huan, T.D.; Ramprasad, R. Electronic structure of polyethylene: Role of chemical, morphological and interfacial complexity. Sci. Rep. 2017, 7, 6128. [Google Scholar] [CrossRef]
- National Library of Medicine—National Institutes of Health, NIH U.S. National Library of Medicine. Limonene. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Limonene (accessed on 29 August 2019).
- National Library of Medicine—National Institutes of Health, NIH U.S. National Library of Medicine. Acetophenone. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Acetophenone (accessed on 29 August 2019).
- Karlsson, M.; Xu, X.; Gaska, K.; Hillborg, H.; Gubanski, S.; Gedde, U.W. DC Conductivity Measurements of LDPE: Influence of Specimen Preparation Method and Polymer Morphology. Proc. Nord. Insul. Symp. 2017, 25. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Pourrahimi, A.M.; Olsson, R.T.; Hedenqvist, M.S.; Gedde, U.W. Influence of nanoparticle surface treatment on particle dispersion and interfacial adhesion in low-density polyethylene/aluminium oxide nanocomposites. Eur. Polym. J. 2015, 66, 67–77. [Google Scholar] [CrossRef]
- Liu, D.; Pourrahimi, A.M.; Pallon, L.K.H.; Andersson, R.L.; Hedenqvist, M.S.; Gedde, U.W.; Olsson, R.T. Morphology and properties of silica-based coatings with different functionalities for Fe3, ZnO and Al2O3 nanoparticles. RSC Adv. 2015, 5, 48094. [Google Scholar] [CrossRef] [Green Version]
- Gedde, U.W. Polymer Physics; Springer: Stockholm, Sweden, 1999. [Google Scholar]
- Wunderlich, B.; Bauer, H. Heat Capacities of Linear High Polymers. Adv. Polym. Sci. 1970, 7, 151. [Google Scholar] [CrossRef]
- Wunderlich, B. Heat of Fusion of Polyethylene. J Polym. Sci. Part A 1967, 2, 5. [Google Scholar] [CrossRef]
- Panayiotis, J.K.; Baptiste, M.J. Thermal and Structural Properties of Fusion Related Materials. Available online: http://www-ferp.ucsd.edu/LIB/PROPS/PANOS/al2o3.html (accessed on 29 August 2019).
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Clarendon Press: Oxford, UK, 1986. [Google Scholar]
- Hedenqvist, M.S.; Ohrlander, M.; Palmgren, R.; Albertsson, A. Multi-layer modelling of diffusion of water in acrylamide-grafted aliphatic polyesters. Polym. Eng. Sci. 1998, 38, 1313–1323. [Google Scholar] [CrossRef]
- Hedenqvist, M.S.; Gedde, U.W. Parameters affecting the determination of transport kinetics data in highly swelling polymers above Tg. Polymer 1999, 40, 2381–2393. [Google Scholar] [CrossRef]
- Bakhouya, A.; Brouzi, A.E.; Bouzon, J.; Vergnaud, J.M. Modelling the Process of Desorption of a Liquid from a Sheet of Polymer by Considering the Diffusion and Evaporation and the Change in Dimension. Plast. Rubber Comp. Proc. Appl. 1993, 19, 77. [Google Scholar]
- Nordell, P.; Nilsson, F.; Hedenqvist, M.S.; Hillborg, H.; Gedde, U.W. Water transport in aluminium oxide-poly (ethylene-co-butyl acrylate) nanocomposites. Eur. Polym. J. 2011, 47, 2208–2215. [Google Scholar] [CrossRef]
- Nilsson, F.; Hallstensson, K.; Johansson, K.; Umar, Z.; Hedenqvist, M.S. Predicting Solubility and Diffusivity of Gases in Polymers under High Pressure: N2 in Polycarbonate and Poly(ether-ether-ketone). Ind. Eng. Chem. Res. 2013, 52, 8655–8663. [Google Scholar] [CrossRef]
- Nilsson, F.; Gedde, U.W.; Hedenqvist, M.S. Penetrant diffusion in polyethylene spherulites assessed by a novel off-lattice Monte Carlo technique. Eur. Polym. J. 2009, 45, 3409–3417. [Google Scholar] [CrossRef]








| Sample | Property | |||
|---|---|---|---|---|
| Tm a (°C) | Tc b (°C) | Lc c (nm) | Wc d (%) | |
| PE (powder) b | 112.8 | 98.9 | 8.9 | 46 |
| PE-Hept (extruded) | 111.1 | 99.1 | 8.6 | 50 |
| PE-3wt.%-Al2O3-PNC | 112.3 | 99.1 | 8.7 | 50 |
| PE-6wt.%-Al2O3-PNC | 111.6 | 99.1 | 8.5 | 48 |
| PE-9wt.%-Al2O3-PNC | 111.5 | 99.1 | 8.5 | 47 |
| PE-12wt.%-Al2O3-PNC | 113.0 | 98.9 | 8.9 | 49 |
| PE-3wt.%-C8-Al2O3-PNC | 111.8 | 99.3 | 8.5 | 52 |
| PE-6wt.%-C8-Al2O3-PNC | 111.7 | 99.3 | 8.5 | 53 |
| PE-9wt.%-C8-Al2O3-PNC | 110.9 | 99.5 | 8.3 | 50 |
| PE-12wt.%-C8-Al2O3-PNC | 112.8 | 99.1 | 8.9 | 49 |
| Sample | Solubility (wt.%) | D (Equation (3)) (×10−9 cm2 s−1) | Dc0 (Equation (5)) (×10−9 cm2s−1) |
|---|---|---|---|
| PE (extruded) | 2.1 | 5.1 | 4.3 |
| PE-3wt.%-Al2O3-PNC | 2.1 | 5.6 | 4.4 |
| PE-6wt.%-Al2O3-PNC | 2.3 | 6.1 | 3.8 |
| PE-9wt.%-Al2O3-PNC | 2.4 | 5.9 | 3.9 |
| PE-12wt.%-Al2O3-PNC | 2.5 | 5.8 | 3.4 |
| PE-3wt.%-C8-Al2O3-PNC | 2.1 | 6.3 | 4.5 |
| PE-6wt.%-C8-Al2O3-PNC | 2.2 | 5.8 | 4.2 |
| PE-9wt.%-C8-Al2O3-PNC | 2.3 | 5.7 | 4.1 |
| PE-12wt.%-C8-Al2O3-PNC | 2.4 | 6.1 | 3.9 |
| Sample | Solubility (wt.%) | D (Equation (3)) (×10−9 cm2 s−1) |
|---|---|---|
| PE (extruded) | 12.9 | 29 |
| PE-1wt.%-Al2O3-PNC | 12.6 | 25 |
| PE-3wt.%-Al2O3-PNC | 12.7 | 32 |
| PE-5wt.%-Al2O3-PNC | 12.3 | 26 |
| PE-10wt.%-Al2O3-PNC | 12.0 | 32 |
| PE-1wt.%-C8-Al2O3-PNC | 13.1 | 26 |
| PE-3wt.%-C8-Al2O3-PNC | 12.7 | 31 |
| PE-5wt.%-C8-Al2O3-PNC | 12.7 | 29 |
| PE-10wt.%-C8-Al2O3-PNC | 12.5 | 26 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holder, S.L.; Karlsson, M.E.; Olsson, R.T.; Hedenqvist, M.S.; Nilsson, F. Solubility and Diffusivity of Polar and Non-Polar Molecules in Polyethylene-Aluminum Oxide Nanocomposites for HVDC Applications. Energies 2020, 13, 722. https://doi.org/10.3390/en13030722
Holder SL, Karlsson ME, Olsson RT, Hedenqvist MS, Nilsson F. Solubility and Diffusivity of Polar and Non-Polar Molecules in Polyethylene-Aluminum Oxide Nanocomposites for HVDC Applications. Energies. 2020; 13(3):722. https://doi.org/10.3390/en13030722
Chicago/Turabian StyleHolder, Shima L., Mattias E. Karlsson, Richard T. Olsson, Mikael S. Hedenqvist, and Fritjof Nilsson. 2020. "Solubility and Diffusivity of Polar and Non-Polar Molecules in Polyethylene-Aluminum Oxide Nanocomposites for HVDC Applications" Energies 13, no. 3: 722. https://doi.org/10.3390/en13030722
APA StyleHolder, S. L., Karlsson, M. E., Olsson, R. T., Hedenqvist, M. S., & Nilsson, F. (2020). Solubility and Diffusivity of Polar and Non-Polar Molecules in Polyethylene-Aluminum Oxide Nanocomposites for HVDC Applications. Energies, 13(3), 722. https://doi.org/10.3390/en13030722