Simulating the Effect of Torrefaction on the Heating Value of Barley Straw
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Development
2.2. Torrefaction Process
2.3. Bomb Calorimeter
2.4. Ultimate Analysis
2.5. Scanning Electron Microscopy
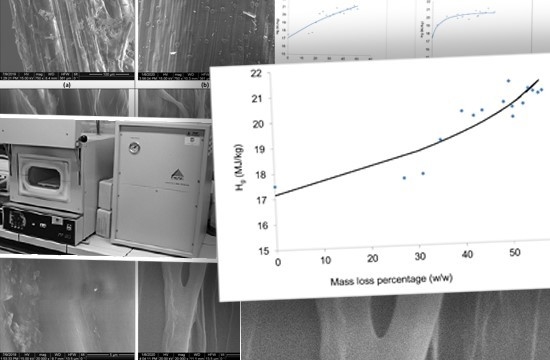
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fragkos, P.; Tasios, N.; Paroussos, L.; Capros, P.; Tsani, S. Energy system impacts and policy implications of the European Intended Nationally Determined Contribution and low-carbon pathway to 2050. Energy Policy 2017, 100, 216–226. [Google Scholar] [CrossRef]
- Corradini, M.; Costantini, V.; Markandya, A.; Paglialunga, E.; Sforna, G. A dynamic assessment of instrument interaction and timing alternatives in the EU low-carbon policy mix design. Energy Policy 2018, 120, 73–84. [Google Scholar] [CrossRef]
- Sung, B.; Park, S.-D. Who Drives the Transition to a Renewable-Energy Economy? Multi-Actor Perspective on Social Innovation. Sustainability 2018, 10, 448. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.H.; Kuo, P.C. Torrefaction and co-torrefaction characterization of hemicellulose, cellulose and lignin as well as torrefaction of some basic constituents in biomass. Energy 2011, 36, 803–811. [Google Scholar] [CrossRef]
- Van der Stelt, M.J.C.; Gerhauser, H.; Kiel, J.H.A.; Ptasinski, K.J. Biomass upgrading by torrefaction for the production of biofuels: A review. Biomass Bioenergy 2011, 35, 3748–3762. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G.; Vassilev, V.S. Advantages and disadvantages of composition and properties of biomass in comparison with coal: An overview. Fuel 2015, 158, 330–350. [Google Scholar] [CrossRef]
- Ntanos, S.; Kyriakopoulos, G.; Chalikias, M.; Arabatzis, G.; Skordoulis, M. Public Perceptions and Willingness to Pay for Renewable Energy: A Case Study from Greece. Sustainability 2018, 10, 687. [Google Scholar] [CrossRef] [Green Version]
- Muench, S. Greenhouse gas mitigation potential of electricity from biomass. J. Clean. Prod. 2015, 103, 483–490. [Google Scholar] [CrossRef]
- Chen, W.; Peng, J.; Bi, X.T. A state-of-the-art review of biomass torrefaction, densification and applications. Renew. Sustain. Energy Rev. 2015, 44, 847–866. [Google Scholar] [CrossRef]
- Dietrich, R.-U.; Albrecht, F.G.; Maier, S.; König, D.H.; Estelmann, S.; Adelung, S.; Bealu, Z.; Seitz, A. Cost calculations for three different approaches of biofuel production using biomass, electricity and CO2. Biomass Bioenergy 2018, 111, 165–173. [Google Scholar] [CrossRef]
- Satpathy, S.K.; Tabil, L.G.; Meda, V.; Naik, S.N.; Prasad, R. Torrefaction of wheat and barley straw after microwave heating. Fuel 2014, 124, 269–278. [Google Scholar] [CrossRef]
- Bridgeman, T.G.; Jones, J.M.; Shield, I.; Williams, P.T. Torrefaction of reed canary grass, wheat straw and willow to enhance solid fuel qualities and combustion properties. Fuel 2008, 87, 844–856. [Google Scholar] [CrossRef]
- Prins, M.J.; Ptasinski, K.J.; Janssen, F.J.J.G. Torrefaction of wood: Part 2: Analysis of products. J. Anal. Appl. Pyrol. 2006, 77, 35–40. [Google Scholar] [CrossRef]
- Arias, B.; Pevida, C.; Fermoso, J.; Plaza, M.G.; Rubiera, F.; Pis, J.J. Influence of Torrefaction on the Grindability and Reactivity of Woody Biomass. Fuel Process. Technol. 2008, 89, 169–175. [Google Scholar] [CrossRef] [Green Version]
- Phanphanich, M.; Mani, S. Impact of Torrefaction on the Grindability and Fuel Characteristics of Forest Biomass. Bioresour. Technol. 2011, 102, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Medic, D.; Darr, M.; Shah, A.; Potter, B.; Zimmerman, J. Effects of Torrefaction Process Parameters on Biomass Feedstock Upgrading. Fuel 2011, 91, 147–154. [Google Scholar] [CrossRef]
- Uemura, Y.; Omar, W.N.; Tsutsui, T.; Yusup, S.B. Torrefaction of Oil Palm Wastes. Fuel 2011, 90, 2585–2591. [Google Scholar] [CrossRef]
- Wannapeera, J.; Fungtammasan, B.; Worasuwannarak, N. Effects of Temperature and Holding Time during Torrefaction on the Pyrolysis Behaviors of Woody Biomass. J. Anal. Appl. Pyrol. 2011, 92, 99–105. [Google Scholar] [CrossRef]
- Bergman, P.C.A.; Boersma, A.R.; Kiel, J.H.A.; Prins, M.J.; Ptasinski, K.J.; Janseen, F.J.J.G. Torrefaction for Entrained Flow Gasification of Biomass. In Biomass for Energy, Industry and Climate Protection: Second World Biomass Conference; Proceedings of the World Conference, Rome, Italy, 10–14 May 2004; van Swaaij, W.P.M., Ed.; ECN-RX; ETA-Renewable Energies: Florence, Italy, 2004; Volume 04-046, pp. 679–682. [Google Scholar]
- Clausen, L.R.; Houbak, N.; Elmegaard, B. Techno-Economic Analysis of a Low CO2 Emission Dimethyl Ether (DME) Plant Based on Gasification of Torrefied Biomass. Energy 2010, 35, 4831–4842. [Google Scholar] [CrossRef] [Green Version]
- Prins, M.J.; Ptasninski, K.J.; Janssen, F.J.J.G. More Efficient Biomass Gasification via Torrefaction. Energy 2006, 31, 3458–3470. [Google Scholar] [CrossRef]
- Deng, J.; Wang, G.J.; Kuang, J.H.; Zhang, Y.-L.; Luo, Y.H. Pretreatment of Agriculture Residues for Co-Gasification via Torrefaction. J. Anal. Appl. Pyrol. 2009, 86, 331–337. [Google Scholar] [CrossRef]
- Pach, M.; Zanzi, R.; Bjornbom, E. Torrefied Biomass a Substitute for Wood and Charcoal. In Proceedings of the 6th Asia-Pacific International Symposium on Combustion and Energy Utilization, Kuala Lumpur, Malaysia, 20–22 May 2002. [Google Scholar]
- Tumuluru, J.S.; Sokhansanj, S.; Hess, J.R.; Wright, C.T.; Boardman, R.D. A Review on Biomass Torrefaction Process and Product Properties for Energy Applications. Ind. Biotechnol. 2011, 7, 384–401. [Google Scholar] [CrossRef] [Green Version]
- Bridgeman, T.G.; Jones, J.M.; Williams, A.; Waldron, D.J. An Investigation of the Grindability of the Torrefied of Energy Crops. Fuel 2010, 89, 3911–3918. [Google Scholar] [CrossRef] [Green Version]
- Felfli, F.F.; Luengo, C.A.; Suarez, J.A.; Beaton, P.A. Wood Briquette Torrefaction. Energy Sustain. Dev. 2005, 9, 19–22. [Google Scholar] [CrossRef]
- Acharjee, T.C.; Coronella, C.J.; Vasquez, V.R. Effect of Thermal Pretreatment on Equilibrium Moisture Content of Lignocellulosic Biomass. Bioresour. Technol. 2011, 102, 4849–4854. [Google Scholar]
- Repellin, V.; Govin, A.; Rolland, M.; Guyonment, R. Energy Required for Fine Grinding of Torrefied Wood. Biomass Bioenergy 2010, 34, 923–930. [Google Scholar] [CrossRef] [Green Version]
- Yan, W.; Acharjee, T.C.; Coronella, C.J.; Vasquez, V.R. Thermal Pretreatment of Lignocellulosic Biomass. Environ. Prog. 2009, 28, 435–440. [Google Scholar] [CrossRef]
- Mobini, M.; Meyer, J.C.; Trippe, F.; Sowlati, T.; Fröhling, M.; Schultmann, F. Assessing the integration of torrefaction into wood pellet production. J. Clean. Prod. 2014, 78, 216–225. [Google Scholar] [CrossRef]
- Pentananunt, R.; Rahman, A.N.M.M.; Bhattacharya, S.C. Upgrading of Biomass by Means of Torrefaction. Energy 1990, 15, 1175–1179. [Google Scholar] [CrossRef]
- Ge, L.; Zhang, Y.; Wang, Z.; Zhou, J.; Cen, K. Effects of Microwave irradiation Treatment on Physicochemical Characteristics of Chinese Low-Rank Coals. Energy Convers. Manag. 2013, 71, 84–91. [Google Scholar] [CrossRef]
- Chaouch, M.; Petrissans, M.; Petrissans, A.; Gerardin, P. Use of Wood Elemental Composition to Predict Heat Treatment Intensity and Decay Resistance Different softwood and Hardwood Species. Polym. Degrad. Stab. 2010, 95, 2255–2259. [Google Scholar] [CrossRef]
- Nhuchhen, D.R.; Basu, P.; Acharya, B. A Comprehensive Review on Biomass Torrefaction. Int. J. Renew. Energy Biofuels 2014, 2014, 1–56. [Google Scholar] [CrossRef]
- International Organization for Standardization ISO 1716:2018 Reaction to Fire Tests for Products—Determination of the Gross Heat of Combustion (Calorific Value). 2018. Available online: https://www.iso.org/standard/70177.html (accessed on 20 December 2019).
- Brasch, D.J.; Free, K.W. Prehydrolysis-kraft pulping of Pinus radiata grown in New Zealand. Tappi 1965, 48, 245–248. [Google Scholar]
- Overend, R.; Chornet, E. Fractionation of lignocellulosics by steam-aqueous pretreatments. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1987, 321, 523–536. [Google Scholar]
- Kim, Y.H.; Na, B.I.; Ahn, B.J.; Lee, H.W.; Lee, J.W. Optimal condition of torrefaction for high energy density solid fuel of fast growing tree species. Korean J. Chem. Eng. 2015, 32, 1547–1553. [Google Scholar] [CrossRef]
- Sidiras, D.; Batzias, F.; Ranjan, R.; Tsapatsis, M. Simulation and optimization of batch autohydrolysis of wheat straw to monosaccharides and oligosaccharides. Bioresour. Technol. 2011, 102, 10486–10492. [Google Scholar] [CrossRef] [PubMed]
- Aguado, R.; Cuevas, M.; Perez-Villarejo, L.; Martínez-Cartas, M.L.; Sanchez, S. Upgrading almond-tree pruning as a biofuel via wet torrefaction. Renew. Energy 2020, 145, 2091–2100. [Google Scholar] [CrossRef]
- Zhang, C.; Ho, S.-H.; Chen, W.-H.; Xie, Y.; Liu, Z.; Chang, J.S. Torrefaction performance and energy usage of biomass wastes and their correlations with torrefaction severity index. Appl. Energy 2018, 220, 598–604. [Google Scholar] [CrossRef]
- Campbell, W.A.; Coller, A.; Evitts, R.W. Comparing severity of continuous torrefaction for five biomass with a wide range of bulk density and particle size. Renew. Energy 2019, 141, 964–972. [Google Scholar] [CrossRef]
- Silveira, E.A.; Gustavo, L.; Galva, O.; Sa, I.A.; Silva, B.F.; Macedo, L.; Rousset, P.; Caldeira-Pires, A. Effect of torrefaction on thermal behavior and fuel properties of Eucalyptus grandis macro-particulates. J. Therm. Anal. Calorim. 2019, 138, 3645–3652. [Google Scholar] [CrossRef]
- Xin, S.; Huang, F.; Liu, X.; Mi, T.; Xu, Q. Torrefaction of herbal medicine wastes: Characterization of the physicochemical properties and combustion behaviors. Bioresour. Technol. 2019, 287, 121408. [Google Scholar] [CrossRef] [PubMed]
- Friedl, A.E.; Padouvas, H.R.; Varmuza, K. Prediction of heating values of biomass fuel from elemental composition. Anal. Chim. Acta 2005, 544, 191–198. [Google Scholar] [CrossRef]
- Bach, Q.-V.; Skreiberg, Ø. Upgrading biomass fuels via wet torrefaction: A review and comparison with dry torrefaction. Renew. Sustain. Energy Rev. 2016, 54, 665–677. [Google Scholar] [CrossRef]
- Cahyanti, M.N.; Doddapaneni, T.R.K.C.; Kikas, T. Biomass torrefaction: An overview on process parameters, economic and environmental aspects and recent advancements. Bioresour. Technol. 2020, 301, 122737. [Google Scholar] [CrossRef]








| Count | t (min) | R0 | LogR0 |
|---|---|---|---|
| 1 | 15 | 36,010 | 4.56 |
| 2 | 17.5 | 332,910 | 5.52 |
| 3 | 20 | 1,724,755 | 6.24 |
| 4 | 22.5 | 5,098,025 | 6.71 |
| 5 | 25 | 8,922,535 | 6.95 |
| 6 | 27.5 | 8,949,697 | 6.95 |
| 7 | 30 | 13,005,480 | 7.11 |
| 8 | 32.5 | 10,659,469 | 7.03 |
| 9 | 35 | 15,435,573 | 7.19 |
| 10 | 37.5 | 16,313,425 | 7.21 |
| 11 | 40 | 16,032,904 | 7.21 |
| 12 | 42.5 | 22,099,405 | 7.34 |
| 13 | 45 | 18,959,522 | 7.28 |
| 14 | 47.5 | 19,904,975 | 7.30 |
| 15 | 50 | 16,903,655 | 7.23 |
| Percentages (% wt. Dry Basis) | Untreated Barley Straw | Torrefied Barley Straw | Analytical Method |
|---|---|---|---|
| Proximate analysis (wt. %) | |||
| Moisture Content | 6.0 | 3.5 | ISO 18134-1 |
| Volatile Matter | 74.3 | 62.5 | ISO 18123 |
| Ash | 8.4 | 16.1 | ISO 18122 |
| Ultimate analysis (wt. %) | |||
| Carbon | 45.5 | 57.5 | ISO 16948 |
| Hydrogen | 5.5 | 4.1 | ISO 16948 |
| Nitrogen | 0.99 | 1.6 | ISO 16948 |
| Oxygen | 47.9 | 36.4 | by difference |
| Sulfur | 0.11 | 57.5 | ISO 16994 |
| Model A1 | Model A2 | Model A3 | |
|---|---|---|---|
| y0 | 101.10 | 80.23 | 70.58 |
| k | 0.0450 | 1.739·10–7 | 7.778·10–8 |
| ye | 36.76 | 45.32 | 38.35 |
| SEE | 3.637 | 7.183 | 2.596 |
| Model B1 | Model B2 | Model B3 | |
|---|---|---|---|
| Hg0 | 17.11 | 17.53 | 17.55 |
| k1 | 0.0006334 | 1.020·10–7 | 1.000·10–7 |
| Hge | 30.87 | 21.18 | 21.19 |
| SEE | 0.6182 | 0.3344 | 0.3479 |
| Materials | HHV (MJ/kg) | Solid Residue Yield, y (% wt.) | Enhancement Factor, EF | Energy Yield, EY (%) | References |
|---|---|---|---|---|---|
| Almond-tree pruning | 17.6 | [28] | |||
| Almond-tree pruning pretreated by wet torrefaction | 24 | 57.1 | 1.36 | 77.9 | [28] |
| Barley straw | 17.7 | [11] | |||
| Barley straw torrefied | 21.5 | 44.1 | 1.21 | 53.6 | [11] |
| Barley straw | 17.5 | This study | |||
| Barley straw torrefied | 21.3 | 49.9 | 1.22 | 60.7 | This study |
| Eucalyptus grandis | 20.1 | [43] | |||
| Eucalyptus grandis torrefied | 25.0 | 65.2 | 1.24 | 81.0 | [43] |
| Herbal medicine wastes | 19 | [44] | |||
| Herbal medicine wastes torrefied | 20.3 | 82.1 | 1.07 | 87.7 | [44] |
| Microalga residue | 12.7 | [41] | |||
| Microalga residue torrefied | 17.3 | 68.0 | 1.36 | 92.6 | [41] |
| Spent coffee grounds | 21.8 | [41] | |||
| Spent coffee grounds torrefied | 29.8 | 72.4 | 1.37 | 98.9 | [41] |
| Spruce | 20.3 | [42] | |||
| Spruce char | 21.2 | 91.5 | 1.04 | 95.6 | [42] |
| Wheat straw | 17.8 | [11] | |||
| Wheat straw torrefied | 20.5 | 64 | 1.15 | 73.7 | [11] |
| Wheat straw | 19 | [42] | |||
| Wheat straw char | 20.1 | 84.8 | 1.06 | 89.7 | [42] |
| Willow | 20.1 | [42] | |||
| Willow char | 21.2 | 87.1 | 1.05 | 91.9 | [42] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sidiras, D.K.; Nazos, A.G.; Giakoumakis, G.E.; Politi, D.V. Simulating the Effect of Torrefaction on the Heating Value of Barley Straw. Energies 2020, 13, 736. https://doi.org/10.3390/en13030736
Sidiras DK, Nazos AG, Giakoumakis GE, Politi DV. Simulating the Effect of Torrefaction on the Heating Value of Barley Straw. Energies. 2020; 13(3):736. https://doi.org/10.3390/en13030736
Chicago/Turabian StyleSidiras, Dimitrios K., Antonios G. Nazos, Georgios E. Giakoumakis, and Dorothea V. Politi. 2020. "Simulating the Effect of Torrefaction on the Heating Value of Barley Straw" Energies 13, no. 3: 736. https://doi.org/10.3390/en13030736
APA StyleSidiras, D. K., Nazos, A. G., Giakoumakis, G. E., & Politi, D. V. (2020). Simulating the Effect of Torrefaction on the Heating Value of Barley Straw. Energies, 13(3), 736. https://doi.org/10.3390/en13030736