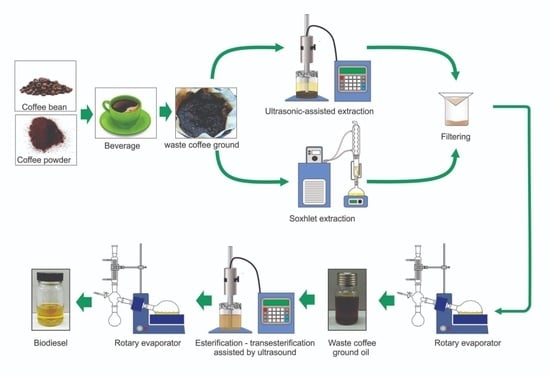
1. Introduction
The limited reserves and increasing price of fossil fuels, as well as the adverse impact of fossil fuel combustion on climate change, has motivated researchers to find an alternative source of energy, e.g., renewable fuel sources [
1,
2,
3]. Biodiesel is a renewable fuel consisting of a mixture of mono-alkyl esters and long-chain fatty acids, and is non-toxic, biodegradable and can be used in diesel engines with minimal modification [
4,
5,
6]. Biodiesel is produced from different sources including edible oils, non-edible oils, and animal fats [
7,
8,
9,
10,
11]. Currently, biodiesel production costs are calculated to be 4.4 times the cost of petroleum-derived diesel production [
12]. Given that the current commercial biodiesel feedstocks are of edible oil origins such as palm and soy, government intervention in the biodiesel market will complicate the effects on food security. The use of WCG oil as biodiesel feedstock promotes biodiesel production from an alternative resource while reducing the issue of landfilling with food waste, which is prominent globally.
Coffee is the world’s second-largest traded liquid commodity, after oil, with approximately 8 million tons of coffee produced each year [
13]. A large amount of heat energy is used to convert green coffee beans into brown roasted beans in the brewing process, generating large amounts of volatile organic compounds (VOCs) [
14]. The enormous demand for this beverage also produces a large quantity of residual waste after brewing. Every 1 ton of coffee beans produces 650 kg of coffee residue after brewing, known as WCG [
15]. The global coffee industry produced an estimate of 9.34 million tons of waste in 2017, which was either incinerated, dumped in landfills or composted [
16]. Every year Australia produces an estimated 75,000 tonnes of used ground coffee waste, and 93% of cafes send their WCGs to landfill. The annual domestic coffee consumption in Australia has reached almost 1.9 million 60 kg bags. On average, Australians consumed around 1.92 kilograms of coffee per person in 2017 [
17]. The grounds that are used to make coffee are used only once and then immediately discarded. With rising rates of consumption, waste residues from the coffee industry (by-products from harvesting, processing, roasting and brewing stages of coffee production and processing) represent a challenge to worldwide directives aiming to reduce landfill volume. The inherent toxicity of several constituents within coffee also presents an environmental contamination concern [
18]. Additionally, waste coffee grounds contribute towards the huge financial cost on taxpayers for running and maintaining landfills. Therefore, a combined solution of collection and reuse of WCG for alternate energy production would be beneficial to the coffee industry. WCG contains 15%–20% of lipid depending on the extraction technologies, which can be used as a source of bioenergy.
Production of oil from non-edible sources such as WCG can also help overcome the food versus fuel dispute [
19]. The abundance of WCGs would also make it a readily available feedstock with a significantly lower production cost than edible oils [
20]. WCG after oil extraction has also been identified as a suitable material for the production of garden fertiliser, feedstock for ethanol production, biogas production and fuel pellets [
21]. However, the use of WCG oil for biodiesel production is still relatively new and requires further research before commercialisation.
This study aims to recover oil from WCG through an ultrasonic-assisted process and to convert it into biodiesel to reduce the volume of waste to landfill; reducing the greenhouse gas emissions associated with coffee waste in landfills. The WCG was subjected to ultrasonic-assisted oil extraction and compared with the Soxhlet extraction process before transesterification was used to produce WCG biodiesel by ultrasonication. The effect of extraction solvent, solvent to WCG ratio, ultrasonic power and ultrasonication period were studied to optimise oil extraction from the WCG. Further optimisation of parameters such as methanol to oil molar ratio, catalyst concentration, ultrasonic power and reaction time were done using Response Surface Methodology (RSM) to obtain the highest ester yield. RSM correlates the relationship between different response variables [
22]. The effect of independent variables is determined by RSM which also creates a mathematical model which can be used for evaluating other relevant variables. The physicochemical properties of the obtained biodiesel were measured and compared with ASTM D6751 and EN 14214 biodiesel standards to determine its successful conversion to biodiesel [
23,
24,
25].
2. Materials and Methods
WCG was obtained from a local store which was then oven-dried at 60° C. High purity analytical grade chemicals (Sigma–Aldrich) and solvents including 2-propanol (purity 99.7%), n-hexane (purity 99%), methanol (purity 99.8%), and potassium hydroxide were used to extract oil and convert into biodiesel.
2.1. Moisture
The moisture content of WCG was determined by measuring the mass before and after the drying of the collected samples. Drying was conducted in an electric oven (Tech-lab, stainless steel forced-air convection oven FAC-138SS) at 100° C for 36 h. Mass of WCG was weighed before drying and at intervals of 12 h. It was found that the mass stopped changing after 36 h which indicates the sample had dried. The following equation was used to determine the moisture content.
where
(g) is the changes between the final and initial mass of the sample,
(g) is the initial mass of WCG.
The moisture content of WCG was found to be 20%. For calculating more accurate oil yields, the mass of WCG was only weighed after the drying process.
Table 1 shows the change of mass of WCG as drying time increases. Mass of WCG remained constant after the 24 h mark.
2.2. Experimental Setup
The reactor used for Soxhlet extraction was equipped with a reflux condenser, one Soxhlet extractor, and a heating mantle. The temperature of the condenser was controlled using a refrigerator cooling bath WiseCircu® (Model: WCR-P8, Daihan Scientific, Gang-Won–Do, South Korea). The experimental setup of WCG Soxhlet extraction is illustrated in
Figure 1a.
The equipment used for ultrasound extraction in this study was a Qsonica Q500-20 sonicator with a 1″ diameter tip (500 W power rating, 20 kHz frequency) ultrasonic probe. The sample was placed in 250 ml beaker made of borosilicate glass as a reactor, where the tip of the probe was fully immersed in the solvent and sample mixture. The probe was placed in the center of the reactor to ensure even ultrasonication of the entire sample. The precaution was taken to ensure that the probe tip was fully immersed to ensure direct sonication of the sample.
Figure 1b shows a schematic of the ultrasonic-assisted extraction setup. The ultrasonication time, amplitude and frequency of ultrasonic waves can be changed using the sonicator system. However, the system was set at a moderate level to avoid energy wastage, deterioration of the sample, and to reduce risk of breaking of the equipment. The ultrasonication can also be set to continuous or pulsed modes. However, to increase the efficiency of the system, a pulsed mode was selected [
26].
2.3. Soxhlet Extraction Method
In this step, 20 g of WCG was weighed in a cellulosic thimble before it was placed in a Soxhlet oil extractor. The oil was extracted using 300 ml of three different types of organic solvents including n-hexane, chloroform and methanol. Among the selected solvents, n-hexane and chloroform are polar in nature whereas methanol is a non-polar solvent. The latent heat of vaporisation of chloroform is the lowest followed by the n-hexane and methanol solvents. These organic solvents increase the yield of oil extraction [
27] compared to other green solvents. The average cycle time was recorded as 15 min. To maintain this extraction cycle time, the temperature was varied based on the chosen solvent. The solvent-oil mixture was placed in a rotary evaporator (IKA RV 10 digital V rotary evaporator with vacuum) at 60 °C to separate the extracted oil. The extraction process was done three times for each solvent type and the average oil extraction yield was calculated by measuring the mass of the dried sample.
2.4. Ultrasonic-assisted Oil Extraction Method
In this step, 10 g of WCG was poured into the 500 ml beaker which also works as a reactor. Selected solvents including n-hexane, chloroform or methanol were added at a ratio 1:20 g/ml into ultrasonicator. The ultrasonic probe was immersed into a sample such that the tip was completely submerged in the solvent mixture. The ultrasonic waves were applied for 5 s with a stop interval of 2 s. Extraction time (25, 37.6, and 50 min) and ultrasonic amplitude (20%, 30%, and 40%) were the other parameters selected for optimisation. The temperature was not selected for optimisation because of continuous compression and rarefaction of the ultrasonic cavitation cycle, which produces heat within the mixture. Filter papers were used for gravitation filtration of the solvent mixture from WCG oil. The rotary evaporator was used to evaporate the sample at 60 °C. The oil yield was calculated using Equation (2).
The molar mass of WCG oil was collected from literature as 862.8 g/mol [
28].
2.5. Response Surface Methodology (RSM)
Design-Expert software version 11 (Stat-Ease Inc., Minneapolis, USA) was used to analyse and optimise experimental data. Analysis of variance (ANOVA) and RSM features of the software were utilised. To optimise the parameters of the oil extraction process, Box–Behnken experimental design was applied. The operating parameters such as the amplitude (X1), reaction time (X2) and n-hexane (X3), were varied to optimise oil yield (Y). The coded and uncoded levels of the Box–Behnken independent variables were presented in
Table 2. The experimental data were analysed in the form of a mathematical model as follows:
where,
Y predicted the yield of WCG crude oil;
Xi is the input independent parameter,
Co and
Ci are the intercept and regression first-order coefficient of the model, regression coefficient among ith and jth input parameters, and the number of input parameters is represented by
k respectively.
Cii is the regression quadratic coefficient of the model for the ith factor.
Cij is the linear coefficient of the model for the interaction between the
ith and
jth factor.
2.6. Two-Step Esterification and Transesterification
The methyl ester was produced using a two-step esterification and trans-esterification process. The esterification process was carried out due to extracted oil having a very high acid value. In this process, the WCG crude oil sample was transferred into a reactor and then mixed with methanol at a molar ratio of 6:1 (methanol to oil). A 1 vol. % H2SO4 catalyst was added into the pre-heated oil (60 °C). An ultrasonication amplitude of 30% was applied for 60 to 105 min. The ultrasonic waves were applied for 5 s with 2-s rest intervals. Following the esterification process, the sample was transferred into a separating funnel. Two distinguishable liquid layers were observed, the top layer consisted of catalyst residues and methanol whereas the bottom layer contained esterified WCG oil.
The esterified oil was placed in a reactor. The required amount of catalyst KOH was weighed into a beaker along with methanol for mixing. Before pouring into the reactor, the mixture was heated and stirred up until the KOH pellets were completely dissolved. The ultrasonic probe was immersed into the mixture in a way that ensured the tip was fully submerged inside the solvent mixture. A similar procedure for ultrasonication as of esterification was followed. The transesterification process was carried out with 1 w/wt% KOH in 6:1 methanol to oil ratio for 30, 45, 60 and 75 min, as a part of the optimisation of reaction time. The temperature was not optimised for the reason explained previously. After transesterification, the separation was carried out by allowing the mixture to settle. The bottom layer (glycerol layer) was removed before washing off the top layer. Washing was done with warm water (60 °C) several times until no impurities were observed in the water. The biodiesel was then placed in a rotary evaporator to remove any remaining moisture. The top biodiesel layer was tested for fatty acid methyl ester (FAME) contents using the Agilent 7890A Gas Chromatograph (GC).
Figure 2 shows the phase separation after transesterification.
2.7. The GC Analysis of the Fatty Acid Composition (FAC)
A GC (Agilent 7890A) fitted with a flame ionisation detector was used to determine the FAME content of the produced biodiesel. Carbon chains (C8-C24) in the FAME layer and linolenic acid methyl ester content of the biodiesel were measured following the EN 14103:2011 standard method with methyl nonadecanoate (internal standard). This method is suitable for use with the GC equipped with HP-INNOWax high-polarity column (length × inner diameter × film thickness: 30 m × 0.25 mm × 0.25 µm, stationary phase: polyethylene glycol). The oven temperature protocol: 2 min constant 60 °C, heated to 200 °C at 10 °C increase per minute, then to 240 °C at 5 °C increase per minute and finally 7 min at 240° C. Helium gas was used as the carrier gas, with a flow rate for helium of 1.5 mL/min. All the FAMEs were chromatographically resolved at the approximate retention time of 25 min. The comparison between the area of methyl ester peaks and internal standard peaks provided the FAME yield (Equation (4)):
where
E signifies the percentage FAME content (%),
the sum of the area of C8:0 to C24:0 peaks,
the peak area of internal standards,
the weight (mg) of methyl nonadecanoate and m is the weight (mg) of the sample.
2.8. FT-IR Analysis
The characterisation of WCG methyl ester was carried out by FT-IR (Perkin Elmer equipped with the MIR TGS detector) in the range 4000–650 cm−1 and analysed with the software program ‘Spectrum’. The resolution was 8 scans and between 8 cm−1 and 4 cm−1.
4. Conclusions
This paper evaluated the recovery of an energy source from a wasted product, i.e., waste coffee grounds, using ultrasonic assistance to produce biodiesel. Use of this feedstock is advantageous as it is abundant, utilises a food waste, and eliminates the side effects of landfill disposal. Some of the salient results are:
Successful production of biofuel oil from the UCG using ultrasonic assistance which was later converted into biodiesel.
The optimum ultrasonic oil extraction conditions were 1:30 g/g of the mass ratio of oil-to-n-hexane, 32% ultrasonic amplitude with a reaction time of 34 min and an achieved oil yield of 17.75 wt.%.
Ultrasonication of WCG during oil extraction reduced the amount of solvent required significantly. This also reduced extraction time and increased extractability compared to the conventional Soxhlet extraction method.
Thus, the study can suggest ultrasonic assistance is a superior method compared to the Soxhlet extraction method. Furthermore, it is also possible to convert waste-to-energy by producing biodiesel from WCG. Further studies are required to evaluate the produced biodiesel as a substitute for petro-diesel.