Hydrothermal Carbonization of Chemical and Biological Pulp Mill Sludges
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
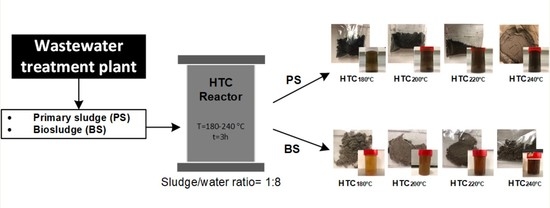
2.2. Hydrothermal Carbonization
2.3. Analytical Methods
3. Results
3.1. Properties of the Sludge Feedstock and Hydrochar
3.2. Inorganic Element Characterization
3.3. Morphology Analysis
3.4. Surface Characterization
3.5. X-ray Diffractograms
3.6. HTC Liquor Characterization
4. Conclusions
- Dehydration and demethylation reactions are the dominant pathways with increasing hydrothermal carbonization temperatures for both biosludge and primary sludge samples, while the role of decarboxylation had a relatively small effect.
- In general, sludge from pulp and paper mills contains a significant amount of inorganic compounds, such as aluminum, silica, zinc and potassium. However, the samples reported lower concentrations than the Finnish legislation limits for their possible soil applications.
- The backscattered electron images analysis showed fibrous and amorphous particle morphologies for primary sludge and biosludge, respectively. The materials were degraded to irregular cracked and shorter fibers for primary sludge and fragmented particles for biosludge with hydrothermal carbonization reaction severity.
- X-ray diffractograms reported a crystallinity size of 1 nm for biosludge with mainly peaks of calcite. A crystallinity size of about 4.6 nm for primary sludge was found with high peaks of crystalline cellulose. FTIR analysis showed that the main organic component of the sludges was cellulose due to a large band associated with C-O- linkages and to a lesser extent, lignin.
- The liquor produced in the hydrothermal treatment showed higher amounts of organic compounds for all primary sludge samples, mainly due to its higher fibrous content and less mineralized material. Inorganic material tends to increase at lower hydrothermal treatment temperatures, mainly at 180 °C for biosludge and 200 °C for primary sludge due to minerals transferred from the solid phase.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Puettmann, M.; Sahoo, K.; Wilson, K.; Oneil, E. Life cycle assessment of biochar produced from forest residues using portable systems. J. Clean. Prod. 2020, 250, 119564. [Google Scholar] [CrossRef]
- Mendoza Martinez, C.L.C.L.; Sermyagina, E.; de Oliveira Carneiro, A.C.; Vakkilainen, E.; Cardoso, M.; de Cassia Oliveira Carneiro, A.; Vakkilainen, E.; Cardoso, M. Production and characterization of coffee-pine wood residues briquettes as an alternative fuel for local firing systems in Brazil. Biomass Bioenergy 2019, 123, 70–77. [Google Scholar] [CrossRef]
- Mendoza Martinez, C.L.; Sermyagina, E.; Saari, J.; de Silva Jesus, M.; Cardoso, M.; de Matheus Almeida, G.; Vakkilainen, E. Hydrothermal carbonization of lignocellulosic agro-forest based biomass residues. Biomass Bioenergy 2021, 147, 106004. [Google Scholar] [CrossRef]
- Veluchamy, C.; Kalamdhad, A.S. Biochemical methane potential test for pulp and paper mill sludge with different food/microorganisms ratios and its kinetics. Int. Biodeterior. Biodegrad. 2017, 117, 197–204. [Google Scholar] [CrossRef]
- CANMET. Pulp and Paper Sludge to Energy—Preliminary Assessment of Technologies, Canada; CANMET Energy Technology Center: Ottawa, ON, Canada, 2005. [Google Scholar]
- Simão, L.; Hotza, D.; Raupp-Pereira, F.; Labrincha, J.A.; Montedo, O.R.K. Wastes from pulp and paper mills-A review of generation and recycling alternatives. Ceramica 2018, 64, 443–453. [Google Scholar] [CrossRef] [Green Version]
- Monte, M.C.; Fuente, E.; Blanco, A.; Negro, C. Waste management from pulp and paper production in the European Union. Waste Manag. 2009, 29, 293–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mäkelä, M.; Watkins, G.; Pöykiö, R.; Nurmesniemi, H.; Dahl, O. Utilization of steel, pulp and paper industry solid residues in forest soil amendment: Relevant physicochemical properties and heavy metal availability. J. Hazard. Mater. 2012, 207, 21–27. [Google Scholar] [CrossRef]
- Saha, N.; Saba, A.; Saha, P.; McGaughy, K.; Franqui-Villanueva, D.; Orts, W.J.; Hart-Cooper, W.M.; Toufiq Reza, M. Hydrothermal carbonization of various paper mill sludges: An observation of solid fuel properties. Energies 2019, 12, 858. [Google Scholar] [CrossRef] [Green Version]
- Mäkelä, M.; Benavente, V.; Fullana, A. Hydrothermal carbonization of lignocellulosic biomass: Effect of process conditions on hydrochar properties. Appl. Energy 2015, 155, 576–584. [Google Scholar] [CrossRef]
- Saari, J.; Kuparinen, K.; Sermyagina, E.; Vakkilainen, E.; Kaikko, J.; Sergeev, V. Effect of integration method and carbonization temperature on the performance of an integrated hydrothermal carbonization and CHP plant. BioResources 2019, 13, 5080–5110. [Google Scholar]
- Stemann, J.; Putschew, A.; Ziegler, F. Hydrothermal carbonization: Process water characterization and effects of water recirculation. Bioresour. Technol. 2013, 143, 139–146. [Google Scholar] [CrossRef]
- Wirth, B.; Reza, T.; Mumme, J. Influence of digestion temperature and organic loading rate on the continuous anaerobic treatment of process liquor from hydrothermal carbonization of sewage sludge. Bioresour. Technol. 2015, 198, 215–222. [Google Scholar] [CrossRef]
- Jaria, G.; Silva, C.P.; Ferreira, C.I.A.; Otero, M.; Calisto, V. Sludge from paper mill effluent treatment as raw material to produce carbon adsorbents: An alternative waste management strategy. J. Environ. Manag. 2017, 188, 203–211. [Google Scholar] [CrossRef] [Green Version]
- Gorzin, F.; Bahri Rasht Abadi, M.M. Adsorption of Cr(VI) from aqueous solution by adsorbent prepared from paper mill sludge: Kinetics and thermodynamics studies. Adsorpt. Sci. Technol. 2018, 36, 149–169. [Google Scholar] [CrossRef]
- Pöykiö, R.; Watkins, G.; Dahl, O. Characterization of primary and secondary wastewater treatment sludge from a pulp and board mill complex to evaluate the feasibility of utilization as a soil amendment agent and a fertilizer product. J. Bioresour. Bioprod. 2018, 3, 88–95. [Google Scholar]
- Fahim, S.; Nisar, N.; Ahmad, Z.; Asghar, Z.; Said, A.; Atif, S.; Ghani, N.; Qureshi, N.; Soomro, G.A.; Iqbal, M.; et al. Managing paper and pulp industry by-product waste utilizing sludge as a bio-fertilizer. Pol. J. Environ. Stud. 2019, 28, 83–90. [Google Scholar] [CrossRef]
- Xu, J.; Liao, Y.; Yu, Z.; Cai, Z.; Ma, X.; Dai, M.; Fang, S. Co-combustion of paper sludge in a 750 t/d waste incinerator and effect of sludge moisture content: A simulation study. Fuel 2018, 217, 617–625. [Google Scholar] [CrossRef]
- Grimm, A.; Etula, J.; Salh, R.; Kalén, G.; Segerström, M.; Brücher, J.; Söderberg, C.; Soukup, D.; Pfeifer, C.; Larsson, S.H. Slagging and fouling characteristics during co-combustion of Scots pine bark with low-temperature dried pulp and paper mill chemical sludge. Fuel Process. Technol. 2019, 93, 282–294. [Google Scholar] [CrossRef]
- Lin, Y.; Ma, X.; Peng, X.; Hu, S.; Yu, Z.; Fang, S. Effect of hydrothermal carbonization temperature on combustion behavior of hydrochar fuel from paper sludge. Appl. Therm. Eng. 2015, 91, 574–582. [Google Scholar] [CrossRef]
- Mäkelä, M.; Benavente, V.; Fullana, A. Hydrothermal carbonization of industrial mixed sludge from a pulp and paper mill. Bioresour. Technol. 2016, 200, 444–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green. Making Wet Waste Valuable. Available online: https://www.c-green.se/ (accessed on 27 December 2020).
- Mäkelä, M.; Forsberg, J.; Söderberg, C.; Larsson, S.H.; Dahl, O. Process water properties from hydrothermal carbonization of chemical sludge from a pulp and board mill. Bioresour. Technol. 2018, 263, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Song, E.; Park, S.; Kim, H. Upgrading Hydrothermal Carbonization (HTC) Hydrochar from Sewage Sludge. Energies 2019, 12, 2383. [Google Scholar] [CrossRef] [Green Version]
- Sermyagina, E.; Saari, J.; Kaikko, J.; Vakkilainen, E. Hydrothermal carbonization of coniferous biomass: Effect of process parameters on mass and energy yields. J. Anal. Appl. Pyrolysis 2015, 113, 551–556. [Google Scholar] [CrossRef]
- Wilk, M.; Magdziarz, A.; Jayaraman, K.; Szymańska-Chargot, M.; Gökalp, I. Hydrothermal carbonization characteristics of sewage sludge and lignocellulosic biomass. A comparative study. Biomass Bioenergy 2019, 120, 166–175. [Google Scholar] [CrossRef]
- Mohammed, I.S.; Na, R.; Kushima, K.; Shimizu, N. Investigating the effect of processing parameters on the products of hydrothermal carbonization of corn stover. Sustainability 2020, 12, 5100. [Google Scholar] [CrossRef]
- Li, W.; Li, Q.; Zhang, Y.; Meng, A. Ashing temperature’s impact on the characteristics of biomass ash. Appl. Mech. Mater. 2013, 261–262, 217–223. [Google Scholar] [CrossRef]
- Freitas, A.J.; Costa, A.C.S.; Oliveira, A.C.; Pereira, B.L.C.; Rocha, M.F.V.; Carneiro, A.C.O. Efeito da Pressão e do Tempo de Compactação nas Propriedades de Briquetes de Resíduos Madeireiros de Paricá. Nativa 2016, 4, 380–385. [Google Scholar] [CrossRef] [Green Version]
- Meyer, T.; Amin, P.; Allen, D.G.; Tran, H. Dewatering of pulp and paper mill biosludge and primary sludge. J. Environ. Chem. Eng. 2018, 6, 6317–6321. [Google Scholar] [CrossRef]
- Dyjakon, A.; Noszczyk, T.; Smȩdzik, M. The influence of torrefaction temperature on hydrophobic properties ofwaste biomass from food processing. Energies 2019, 12, 4609. [Google Scholar] [CrossRef] [Green Version]
- Falco, C.; Baccile, N.; Titirici, M.-M. Morphological and structural differences between glucose, cellulose and lignocellulosic biomass derived hydrothermal carbons. Green Chem. 2011, 13, 3273–3281. [Google Scholar] [CrossRef] [Green Version]
- Mäkelä, M.; Yoshikawa, K. Simulating hydrothermal treatment of sludge within a pulp and paper mill. Appl. Energy 2016, 173, 177–183. [Google Scholar] [CrossRef]
- Mendoza Martinez, C.L.; Saari, J.; Melo, Y.; Cardoso, M.; de Almeida, G.M.; Vakkilainen, E. Evaluation of thermochemical routes for the valorization of solid coffee residues to produce biofuels: A Brazilian case. Renew. Sustain. Energy Rev. 2021, 137, 110585. [Google Scholar]
- Engida, T.; Mekonnen, A.; Wu, J.M.; Xu, D.; Wu, Z.B. Review paper on beverage agro-industrial wastewater treatment plant bio-sludge for fertilizer potential in Ethiopa. Appl. Ecol. Environ. Res. 2020, 18, 33–57. [Google Scholar] [CrossRef]
- Timpe, R.C.; Mann, M.D.; Pavlish, J.H.; Louie, P.K.K. Organic sulfur and hap removal from coal using hydrothermal treatment. Fuel Process. Technol. 2001, 73, 127–141. [Google Scholar] [CrossRef]
- Gendek, A. Combustion heat and calorific value of the mix of sawdust and cones of common pine (Pinus sylvestris L.). Ann. Warsaw Univ. Life Sci.-SGGW Agric. 2015, 66, 137–144. [Google Scholar]
- Huang, R.; Tang, Y. Speciation Dynamics of Phosphorus during (Hydro)Thermal Treatments of Sewage Sludge. Environ. Sci. Technol. 2015, 49, 14466–14474. [Google Scholar] [CrossRef]
- Aho, M.; Yrjas, P.; Taipale, R.; Hupa, M.; Silvennoinen, J. Reduction of superheater corrosion by co-firing risky biomass with sewage sludge. Fuel 2010, 89, 2376–2386. [Google Scholar] [CrossRef]
- Méndez, A.; Fidalgo, J.M.; Guerrero, F.; Gascó, G. Characterization and pyrolysis behaviour of different paper mill waste materials. J. Anal. Appl. Pyrolysis 2009, 86, 66–67. [Google Scholar] [CrossRef]
- Devi, P.; Saroha, A.K. Effect of temperature on biochar properties during paper mill sludge pyrolysis. Int. J. ChemTech Res. 2013, 5, 682–687. [Google Scholar]
- Niinipuu, M.; Latham, K.G.; Boily, J.F.; Bergknut, M.; Jansson, S. The impact of hydrothermal carbonization on the surface functionalities of wet waste materials for water treatment applications. Environ. Sci. Pollut. Res. 2020, 27, 24369–24379. [Google Scholar] [CrossRef]
- Bai, K.; Hao, J.; Yang, Y.; Qian, A. The effect of hydrothermal temperature on the properties of SBA-15 materials. Heliyon 2020, 6, e04436. [Google Scholar] [CrossRef]
- Coimbra, R.N.; Calisto, V.; Ferreira, C.I.A.; Esteves, V.I.; Otero, M. Removal of pharmaceuticals from municipal wastewater by adsorption onto pyrolyzed pulp mill sludge. Arab. J. Chem. 2019, 12, 3611–3620. [Google Scholar] [CrossRef] [Green Version]








| Primary Sludge | Biosludge | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Raw | Reaction Temperature (°C) | Raw | Reaction Temperature (°C) | |||||||
| 180 | 200 | 220 | 240 | 180 | 200 | 220 | 240 | |||
| Elemental composition a (wt% dry) | ||||||||||
| C | 40.8 (0.05 ± 0.02) | 43.21 (0.47 ± 0.09) | 43.50 (0.26 ± 0.05) | 44.24 (0.19 ± 0.05) | 44.35 (0.22 ± 0.1) | 44.76 (0.02 ± 0.01) | 46.01 (0.35 ± 0.1) | 46.58 (0.05 ± 0.02) | 52.66 (0.13 ± 0.1) | 52.57 (0.05 ± 0.02) |
| H | 6.30 (0.07 ± 0.02) | 6.34 (0.03 ± 0.01) | 6.27 (0.02 ± 0.01) | 6.26 (0.02 ± 0.01) | 6.03 (0.02 ± 0.01) | 5.80 (0.01 ± 0.01) | 5.66 (0.19 ± 0.1) | 5.91 (0.14 ± 0.1) | 5.97 (0.02 ± 0.01) | 5.70 (0.02 ± 0.01) |
| O | 42.53 (0.09 ± 0.01) | 43.87 (0.4 ± 0.05) | 43.61(0.21 ± 0.05) | 42.62 (0.24 ± 0.1) | 39.63 (0.17 ± 0.07) | 24.02 (0.02 ± 0.01) | 24.63 (0.50 ± 0.05) | 22.43 (0.19 ± 0.08) | 17.32 (0.11 ± 0.09) | 15.59 (0.12 ± 0.1) |
| N | 0.67 (0.01 ± 0.01) | 0.45 (0.02 ± 0.01) | 0.28 (0.08 ± 0.03) | 0.26 (0.27 ± 0.1) | 0.24 (0.03 ± 0.01) | 4.36 (0.01 ± 0.01) | 4.14 (0.04 ± 0.02) | 4.01 (0.01 ± 0.01) | 2.88 (0.01 ± 0.01) | 2.99 (0.01 ± 0.01) |
| S | 0.42 (0.03 ± 0.01) | 0.24 (0.01 ± 0.01) | 0.21 (0.01 ± 0.01) | 0.18 (0.02 ± 0.01) | 0.24 (0.01 ± 0.01) | 2.30 (0.01 ± 0.01) | 2.27 (0.01 ± 0.01) | 2.22 (0.01 ± 0.01) | 2.27 (0.01 ± 0.01) | 0.28 (0.05 ± 0.03) |
| Proximate composition (wt%) | ||||||||||
| MC | 96.8 (0.20 ± 0.05) | 7.01 (0.46 ± 0.1) | 6.81 (0.76 ± 0.08) | 6.41 (0.60 ± 0.05) | 5.81 (0.30 ± 0.1) | 92.18 (0.29 ± 0.02) | 5.65 (0.45 ± 0.1) | 6.39 (1.15 ± 0.1) | 4.94 (0.67 ± 0.01) | 3.20 (0.12 ± 0.09) |
| VM b | 93.42 (0.24 ± 0.07) | 88.40 (0.14 ± 0.05) | 85.71(0.85 ± 0.05) | 86.04 (1.37 ± 0.2) | 81.30 (1.49 ± 0.6) | 69.16 (0.2 ± 0.07) | 63.33 (0.4 ± 0.04) | 56.75 (1.5 ± 0.2) | 55.21 (0.7 ± 0.08) | 52.97 (0.4 ± 0.05) |
| FC b | − | 5.72 (0.54 ± 0.01) | 8.17(0.16 ± 0.07) | 7.51 (0.63 ± 0.1) | 9.20 (0.45 ± 0.06) | 12.06 (0.2 ± 0.03) | 19.50 (0.5 ± 0.01) | 24.41 (1.8 ± 0.1) | 25.88 (1.0 ± 0.05) | 26.16 (1.0 ± 0.07) |
| AC b 550°C | 9.45 (0.46 ± 0.03) | 5.88 (0.84 ± 0.02) | 6.12(0.12 ± 0.02) | 6.44 (0.32 ± 0.1) | 9.49 (1.22 ± 0.05) | 20.99 (1.27 ± 0.01) | 17.17 (0.14 ± 0.09) | 18.83 (1.28 ± 0.2) | 18.90 (1.39 ± 0.05) | 20.87 (0.31 ± 0.05) |
| AC b 815°C | 9.21 (1.49 ± 0.2) | 5.64 (0.68 ± 0.01) | 5.97(0.28 ± 0.09) | 5.74 (0.08 ± 0.01) | 9.25 (1.13 ± 0.1) | 18.77 (0.43 ± 0.1) | 16.71 (0.14 ± 0.07) | 18.02 (1.14 ± 0.09) | 18.09 (1.37 ± 0.04) | 20.21 (0.37 ± 0.08) |
| Heating value (MJ∙kg dry−1) | ||||||||||
| HHV | 17.50 (0.1 ± 0.05) | 18.57 (0.1 ± 0.05) | 18.57(0.1 ± 0.05) | 18.70 (0.1 ± 0.05) | 18.77 (0.1 ± 0.05) | 19.78 (0.1 ± 0.05) | 21.0 (0.1 ± 0.05) | 24.4 (0.1 ± 0.05) | 24.5 (0.1 ± 0.05) | 24.7 (0.1 ± 0.05) |
| LHV | 16.07 (0.1 ± 0.05) | 17.14 (0.1 ± 0.05) | 17.15(0.1 ± 0.05) | 17.28 (0.1 ± 0.05) | 17.41 (0.1 ± 0.05) | 18.5 (0.1 ± 0.05) | 19.7 (0.1 ± 0.05) | 23.1 (0.1 ± 0.05) | 23.1 (0.1 ± 0.05) | 23.5 (0.1 ± 0.05) |
| NHV | 15.12 (0.1 ± 0.05) | 15.75 (0.1 ± 0.05) | 15.81(0.1 ± 0.05) | 16.01 (0.1 ± 0.05) | 26.25 (0.1 ± 0.05) | 17.8 (0.1 ± 0.05) | 18.5 (0.1 ± 0.05) | 21.5 (0.1 ± 0.05) | 21.8 (0.1 ± 0.05) | 22.7 (0.1 ± 0.05) |
| DE [−] | − | 1.06 | 1.06 | 1.07 | 1.07 | − | 1.06 | 1.23 | 1.24 | 1.25 |
| Primary Sludge | Biosludge | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Raw | Reaction Temperature (°C) | Raw | Reaction Temperature (°C) | |||||||
| 180 | 200 | 220 | 240 | 180 | 200 | 220 | 240 | |||
| TC (mg∙L−1) | 698 (0.1 ± 0.05) | 521 (0.1 ± 0.05) | 1342 (0.2 ± 0.04) | 876 (0.1 ± 0.07) | 1146 (0.08 ± 0.05) | 408 (0.09 ± 0.05) | 310 (0.05 ± 0.01) | 266 (0.1 ± 0.05) | 338 (0.7 ± 0.1) | 311 (0.1 ± 0.01) |
| IC (mg∙L−1) | 264 (0.3 ± 0.02) | 54 (0.2 ± 0.03) | 45 (0.1 ± 0.08) | 56 (0.4 ± 0.01) | 4 (0.1 ± 0.02) | 4 (0.1 ± 0.05) | 3 (0.3 ± 0.05) | 3 (0.5 ± 0.01) | 3 (0.2 ± 0.01) | 3 (0.7 ± 0.02) |
| TOC (mg∙L−1) | 434 (0.1 ± 0.03) | 467 (0.1 ± 0.05) | 1297 (0.1 ± 0.05) | 820 (0.6 ± 0.05) | 1142 (0.9 ± 0.04) | 404 (0.3 ± 0.01) | 307 (0.9 ± 0.05) | 263 (0.6 ± 0.05) | 335 (0.6 ± 0.02) | 308 (0.4 ± 0.02) |
| NVR (−) | 1.43 (0.4 ± 0.1) | 2.36 (0.6 ± 0.09) | 2.76 (0.8 ± 0.1) | 3.05 (0.8 ± 0.05) | 2.23 (1 ± 0.08) | 9.78 (0.8 ± 0.05) | 8.93 (0.4 ± 0.01) | 10.07 (0.1 ± 0.05) | 7.27 (0.5 ± 0.01) | 9.88 (1 ± 0.09) |
| COD (mg∙L−1) | 105 (1.2 ± 0.5) | 126 (1.5 ± 0.08) | 345 (1 ± 0.1) | 253 (1.9 ± 0.2) | 331 (1.6 ± 0.02) | 49 (0.9 ± 0.02) | 24 (0.3 ± 0.02) | 43 (1.7 ± 0.01) | 67 (0.9 ± 0.01) | 81 (2 ± 0.5) |
| pH (−) | 7.66 (0.5 ± 0.1) | 5.19 (0.7 ± 0.09) | 4.79 (0.4 ± 0.1) | 4.35 (0.9 ± 0.02) | 4.07 (0.7 ± 0.01) | 8.65 (1.2 ± 0.1) | 7.17 (0.7 ± 0.01) | 6.31 (0.6 ± 0.1) | 7.40 (1.4 ± 0.04) | 6.93 (1 ± 0.09) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendoza Martinez, C.L.; Sermyagina, E.; Vakkilainen, E. Hydrothermal Carbonization of Chemical and Biological Pulp Mill Sludges. Energies 2021, 14, 5693. https://doi.org/10.3390/en14185693
Mendoza Martinez CL, Sermyagina E, Vakkilainen E. Hydrothermal Carbonization of Chemical and Biological Pulp Mill Sludges. Energies. 2021; 14(18):5693. https://doi.org/10.3390/en14185693
Chicago/Turabian StyleMendoza Martinez, Clara Lisseth, Ekaterina Sermyagina, and Esa Vakkilainen. 2021. "Hydrothermal Carbonization of Chemical and Biological Pulp Mill Sludges" Energies 14, no. 18: 5693. https://doi.org/10.3390/en14185693
APA StyleMendoza Martinez, C. L., Sermyagina, E., & Vakkilainen, E. (2021). Hydrothermal Carbonization of Chemical and Biological Pulp Mill Sludges. Energies, 14(18), 5693. https://doi.org/10.3390/en14185693