Flexible Films as Anode Materials Based on rGO and TiO2/MnO2 in Li-Ion Batteries Free of Non-Active Agents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Graphene Oxide (GO)
2.3. Synthesis of Manganese Dioxide (MnO2)
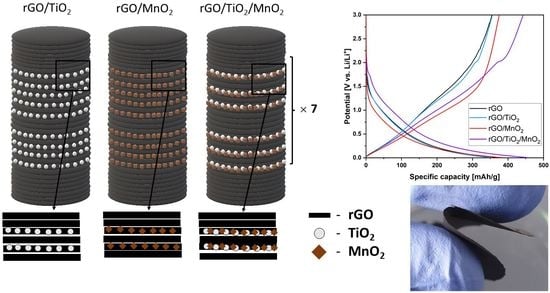
2.4. Thin Film Preparation
2.5. Electrochemical Measurement
2.6. Material Characterization
3. Results and Discussion
3.1. Transmission Electron Microscopy
3.2. Scanning Electron Microscopy
3.3. X-ray Diffraction
3.4. Raman Spectroscopy
3.5. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, W.; Song, M.-S.; Kong, B.; Cui, Y. Flexible and Stretchable Energy Storage: Recent Advances and Future Perspectives. Adv. Mater. 2017, 29, 1603436. [Google Scholar] [CrossRef]
- Lin, L.; Ning, H.; Song, S.; Xu, C.; Hu, N. Flexible Electrochemical Energy Storage: The Role of Composite Materials. Compos. Sci. Technol. 2020, 192, 108102. [Google Scholar] [CrossRef]
- Mo, R.; Rooney, D.; Sun, K. Hierarchical Graphene-Scaffolded Mesoporous Germanium Dioxide Nanostructure for High-Performance Flexible Lithium-Ion Batteries. Energy Storage Mater. 2020, 29, 198–206. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-Ion Battery Materials: Present and Future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Singh, V.; Joung, D.; Zhai, L.; Das, S.; Khondaker, S.I.; Seal, S. Graphene Based Materials: Past, Present and Future. Prog. Mater. Sci. 2011, 56, 1178–1271. [Google Scholar] [CrossRef]
- Peng, Z.; Zhang, H.; Ali, I.; Li, J.; Ding, Y.; Deng, L.; Han, T.; Zhu, H.; Zeng, X.; Cheng, D.; et al. A Rod-on-Tube CoMoO4@hydrogel Composite as Lithium-Ion Battery Anode with High Capacity and Stable Rate-Performance. J. Alloys Compd. 2021, 858, 157648. [Google Scholar] [CrossRef]
- Tariq, Z.; Rehman, S.U.; Zhang, J.; Butt, F.K.; Zhang, X.; Cheng, B.; Zahra, S.; Li, C. Hierarchical Mesoporous Nanoflowers of Zn2VO4 for High Capacity Anode in Lithium Ion Batteries. Mater. Sci. Semicond. Process. 2021, 123, 105549. [Google Scholar] [CrossRef]
- di Lecce, D.; Andreotti, P.; Boni, M.; Gasparro, G.; Rizzati, G.; Hwang, J.-Y.; Sun, Y.-K.; Hassoun, J. Multiwalled Carbon Nanotubes Anode in Lithium-Ion Battery with LiCoO2, Li[Ni1/3Co1/3Mn1/3]O2, and LiFe1/4Mn1/2Co1/4PO4 Cathodes. ACS Sustain. Chem. Eng. 2018, 6, 3225–3232. [Google Scholar] [CrossRef]
- Srinivaas, M.; Wu, C.-Y.; Duh, J.-G.; Hu, Y.-C.; Wu, J.M. Multi-Walled Carbon-Nanotube-Decorated Tungsten Ditelluride Nanostars as Anode Material for Lithium-Ion Batteries. Nanotechnology 2020, 31, 035406. [Google Scholar] [CrossRef]
- Khomenko, V.G.; Barsukov, V.Z.; Doninger, J.E.; Barsukov, I.V. Lithium-Ion Batteries Based on Carbon–Silicon–Graphite Composite Anodes. J. Power Sources 2007, 165, 598–608. [Google Scholar] [CrossRef]
- Yang, M.; Ko, S.; Im, J.S.; Choi, B.G. Free-Standing Molybdenum Disulfide/Graphene Composite Paper as a Binder- and Carbon-Free Anode for Lithium-Ion Batteries. J. Power Sources 2015, 288, 76–81. [Google Scholar] [CrossRef]
- Stoller, M.D.; Park, S.; Zhu, Y.; An, J.; Ruoff, R.S. Graphene-Based Ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior Thermal Conductivity of Single-Layer Graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- He, Q.; Sudibya, H.G.; Yin, Z.; Wu, S.; Li, H.; Boey, F.; Huang, W.; Chen, P.; Zhang, H. Centimeter-Long and Large-Scale Micropatterns of Reduced Graphene Oxide Films: Fabrication and Sensing Applications. ACS Nano 2010, 4, 3201–3208. [Google Scholar] [CrossRef]
- Wu, S.; Yin, Z.; He, Q.; Huang, X.; Zhou, X.; Zhang, H. Electrochemical Deposition of Semiconductor Oxides on Reduced Graphene Oxide-Based Flexible, Transparent, and Conductive Electrodes. J. Phys. Chem. C 2010, 114, 11816–11821. [Google Scholar] [CrossRef]
- Rowden, B.; Garcia-Araez, N. Estimating Lithium-Ion Battery Behavior from Half-Cell Data. Energy Rep. 2021, 7, 97–103. [Google Scholar] [CrossRef]
- Joshi, B.; Samuel, E.; Kim, M.-W.; Kim, K.; Kim, T.-G.; Swihart, M.T.; Yoon, W.Y.; Yoon, S.S. Electrosprayed Graphene Films Decorated with Bimetallic (Zinc-Iron) Oxide for Lithium-Ion Battery Anodes. J. Alloys Compd. 2019, 782, 699–708. [Google Scholar] [CrossRef]
- Dong, Y.; Chen, S.; Liu, J.; Lei, J.; Liu, F.; Yang, W.; Wang, J. Polygonal WS2-Decorated-Graphene Multilayer Films with Microcavities Prepared from a Cheap Precursor as Anode Materials for Lithium-Ion Batteries. Mater. Lett. 2019, 254, 73–76. [Google Scholar] [CrossRef]
- Fang, D.; Ji, Y.; Sun, B.; Bao, R.; Yi, J.; Luo, Z. Cobalt Oxide Nanoparticles Anchored on Discharged-Graphene Film for Lithium-Ion Battery. Solid State Ion. 2019, 340, 115006. [Google Scholar] [CrossRef]
- Dong, L.; Li, M.; Dong, L.; Zhao, M.; Feng, J.; Han, Y.; Deng, J.; Li, X.; Li, D.; Sun, X. Hydrothermal Synthesis of Mixed Crystal Phases TiO2–Reduced Graphene Oxide Nanocomposites with Small Particle Size for Lithium Ion Batteries. Int. J. Hydrogen Energy 2014, 39, 16116–16122. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, S.; Tan, X.; Hou, G.; Zheng, G. TiO2/Graphene Nanocomposites as Anode Materials for High Rate Lithium-Ion Batteries. J. Cent. South Univ. 2014, 21, 1714–1718. [Google Scholar] [CrossRef]
- Xu, T.; Meng, Q.; Fan, Q.; Yang, M.; Zhi, W.; Cao, B. Electrophoretic Deposition of Binder-Free MnO 2 /Graphene Films for Lithium-Ion Batteries. Chin. J. Chem. 2017, 35, 1575–1585. [Google Scholar] [CrossRef]
- Cao, H.; Suib, S.L. Highly Efficient Heterogeneous Photooxidation of 2-Propanol to Acetone with Amorphous Manganese Oxide Catalysts. J. Am. Chem. Soc. 1994, 116, 5334–5342. [Google Scholar] [CrossRef]
- Wei, X.; Zhu, G.; Fang, J.; Chen, J. Synthesis, Characterization, and Photocatalysis of Well-Dispersible Phase-Pure Anatase TiO2 Nanoparticles. Int. J. Photoenergy 2013, 2013, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Radoń, A.; Włodarczyk, P.; Łukowiec, D. Structure, Temperature and Frequency Dependent Electrical Conductivity of Oxidized and Reduced Electrochemically Exfoliated Graphite. Phys. E Low-Dimens. Syst. Nanostruct. 2018, 99, 82–90. [Google Scholar] [CrossRef]
- Challagulla, S.; Tarafder, K.; Ganesan, R.; Roy, S. Structure Sensitive Photocatalytic Reduction of Nitroarenes over TiO2. Sci. Rep. 2017, 7, 8783. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L. Fabrication of β-MnO2/RGO Composite and Its Electrochemical Properties. Int. J. Electrochem. Sci. 2016, 11, 10815–10826. [Google Scholar] [CrossRef]
- Kernazhitsky, L.; Shymanovska, V.; Gavrilko, T.; Puchkovska, G.; Naumov, V.; Khalyavka, T.; Kshnyakin, V.; Chernyak, V.; Baran, J. Optical and Photocatalytic Properties of Titanium–Manganese Mixed Oxides. Mater. Sci. Eng. B 2010, 175, 48–55. [Google Scholar] [CrossRef]
- Cheng, S.; Yang, L.; Chen, D.; Ji, X.; Jiang, Z.; Ding, D.; Liu, M. Phase Evolution of an Alpha MnO2-Based Electrode for Pseudo-Capacitors Probed by in Operando Raman Spectroscopy. Nano Energy 2014, 9, 161–167. [Google Scholar] [CrossRef]
- van de Krol, R.; Goossens, A.; Schoonman, J. Spatial Extent of Lithium Intercalation in Anatase TiO2. J. Phys. Chem. B 1999, 103, 7151–7159. [Google Scholar] [CrossRef]
- Han, Q.; Zhang, W.; Han, Z.; Wang, F.; Geng, D.; Li, X.; Li, Y.; Zhang, X. Preparation of PAN-Based Carbon Fiber@MnO2 Composite as an Anode Material for Structural Lithium-Ion Batteries. J. Mater. Sci. 2019, 54, 11972–11982. [Google Scholar] [CrossRef]










| Film | WEIGHT RATIO | Thickness [µm] | ||
|---|---|---|---|---|
| GO | TiO2 | MnO2 | ||
| rGO | 5 | 0 | 0 | 10.86 |
| rGO/TiO2 | 5 | 1 | 0 | 2.66 |
| rGO/MnO2 | 5 | 0 | 1 | 2.79 |
| rGO/TiO2/MnO2 | 5 | 1 | 1 | 2.29 |
| Film | RS [Ω] | RSEI [Ω] | Rct [Ω] |
|---|---|---|---|
| rGO | 3.24 | - | 228 |
| rGO/TiO2 | 5.07 | - | 481.7 |
| rGO/MnO2 | 3.19 | - | 257.5 |
| rGO/TiO2/MnO2 | 3.28 | 181.1 | 351.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kędzierski, T.; Baranowska, D.; Bęben, D.; Zielińska, B.; Chen, X.; Mijowska, E. Flexible Films as Anode Materials Based on rGO and TiO2/MnO2 in Li-Ion Batteries Free of Non-Active Agents. Energies 2021, 14, 8168. https://doi.org/10.3390/en14238168
Kędzierski T, Baranowska D, Bęben D, Zielińska B, Chen X, Mijowska E. Flexible Films as Anode Materials Based on rGO and TiO2/MnO2 in Li-Ion Batteries Free of Non-Active Agents. Energies. 2021; 14(23):8168. https://doi.org/10.3390/en14238168
Chicago/Turabian StyleKędzierski, Tomasz, Daria Baranowska, Damian Bęben, Beata Zielińska, Xuecheng Chen, and Ewa Mijowska. 2021. "Flexible Films as Anode Materials Based on rGO and TiO2/MnO2 in Li-Ion Batteries Free of Non-Active Agents" Energies 14, no. 23: 8168. https://doi.org/10.3390/en14238168
APA StyleKędzierski, T., Baranowska, D., Bęben, D., Zielińska, B., Chen, X., & Mijowska, E. (2021). Flexible Films as Anode Materials Based on rGO and TiO2/MnO2 in Li-Ion Batteries Free of Non-Active Agents. Energies, 14(23), 8168. https://doi.org/10.3390/en14238168