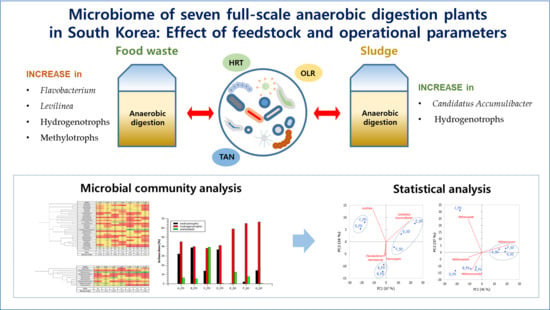
Microbiome of Seven Full-Scale Anaerobic Digestion Plants in South Korea: Effect of Feedstock and Operational Parameters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Full-Scale Anaerobic Digestion Plants Studied and Sample Collection Procedure
2.2. Analysis of Microbiome
2.3. Analytical Methods for Physicochemical Parameters
3. Results and Discussion
3.1. Performance of Full-Scale Anaerobic Digestion Plants
3.2. Microbial Diversity
3.2.1. Bacterial Structure
3.2.2. Archaeal Structure
3.3. Principal Component Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cherubini, F.; Bargigli, S.; Ulgiati, S. Life cycle assessment (LCA) of waste management strategies: Landfill, sorting plant, and incineration. Energy 2009, 34, 2116–2123. [Google Scholar] [CrossRef]
- Evangelisti, S.; Lettieri, P.; Borello, D.; Clift, R. Life cycle assessment of energy from waste via anaerobic digestion: A case study in the UK. Waste Manag. 2014, 34, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Pilli, S.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y. Thermal pretreatment of sewage sludge to enhance anaerobic digestion: A review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 669–702. [Google Scholar]
- Ministry of Environment. The State of Municipal Waste Generation and Treatment in 2012; Ministry of Environment: Seoul, Korea, 2019.
- Angenent, L.T.; Karim, K.; Al-Dahhan, M.H.; Wrenn, B.A.; Domíguez-Espinosa, R. Production of bioenergy and biochemicals from industrial and agricultural wastewater. Trends Biotechnol. 2004, 22, 477–485. [Google Scholar] [CrossRef]
- Hassa, J.; Maus, I.; Off, S.; Pühler, A.; Scherer, P.; Klocke, M.; Schlüter, A. Metagenome, metatranscriptome, and metaproteome approaches unraveled the composition and functional relationships of microbial communities residing in biogas plants. Appl. Microbiol. Biotechnol. 2018, 102, 5045–5063. [Google Scholar] [CrossRef] [Green Version]
- De Vrieze, J.; Saunders, A.M.; He, Y.; Fang, J.; Nielsen, P.H.; Verstraete, W.; Boon, N. Ammonia and temperature determine potential clustering in the anaerobic digestion microbiome. Water Res. 2015, 75, 312–323. [Google Scholar] [CrossRef]
- Kim, E.; Lee, J.; Han, G.; Hwang, S. Comprehensive analysis of microbial communities in full-scale mesophilic and thermophilic anaerobic digesters treating food waste-recycling wastewater. Bioresour. Technol. 2018, 259, 442–450. [Google Scholar]
- Smith, A.L.; Shimada, T.; Raskin, L. A comparative evaluation of community structure in full-scale digesters indicates that two-phase digesters exhibit greater microbial diversity than single-phase digesters. Environ. Sci. Water Res. Technol. 2017, 3, 304–311. [Google Scholar] [CrossRef]
- Kirkegaard, R.H.; McIlroy, S.J.; Kristensen, J.M.; Nierychlo, M.; Karst, S.M.; Dueholm, M.S.; Albertsen, M.; Nielsen, P.H. Impact of immigration on microbial community composition in full-scale anaerobic digesters. Sci. Rep. 2017, 7, 9343. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, M.; Ma, X.; Gao, Q.; Wang, T.; Shi, X.; Zhou, J.; Zuo, J.; Yang, Y. High variations of methanogenic microorganisms drive the full-scale anaerobic digestion process. Environ. Int. 2019, 126, 543–551. [Google Scholar] [CrossRef]
- Yun, Y.M.; Shin, H.S.; Lee, C.K.; Oh, Y.K.; Kim, H.W. Inhibition of residual n-hexane in anaerobic digestion of lipid-extracted microalgal wastes and microbial community shift Environ. Sci. Pollut. Res. 2016, 23, 7138–7145. [Google Scholar] [CrossRef]
- DeLong, E.F. Archaea in coastal marine environments. Proc. Natl. Acad. Sci. USA. 1992, 89, 5685–5689. [Google Scholar] [CrossRef] [Green Version]
- Ovreås, L.; Forney, L.; Daae, F.L.; Torsvik, V. Distribution of bacterioplankton in meromictic Lake Saelenvannet, as determined by denaturing gradient gel electrophoresis of PCR-amplified gene fragments coding for 16S rRNA. Appl. Environ. Microbiol. 1997, 63, 3367–3373. [Google Scholar] [CrossRef] [Green Version]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows the analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Fu, L.; Niu, B.; Wu, S.; Wooley, J. Ultrafast clustering algorithms for metagenomic sequence analysis. Brief. Bioinform. 2012, 13, 656–668. [Google Scholar]
- Cole, J.R.; Wang, Q.; Cardenas, E.; Fish, J.; Chai, B.; Farris, R.J.; Kulam-Syed-Mohideen, A.S.; McGarrell, D.M.; Marsh, T.; Garrity, G.M. The Ribosomal Database Project: Improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 2009, 37, 141–145. [Google Scholar] [CrossRef] [Green Version]
- Letunic, I.; Bork, P. Interactive tree of life (iTOL) v4: Recent updates and new developments. Nucleic Acids Res. 2019, 47, W256–W259. [Google Scholar] [CrossRef] [Green Version]
- APHA. Standard Methods for the Examination of Water and Wastewater, 20th ed.; USA American Public Health Association: Washington, DC, USA, 1998. [Google Scholar]
- Morales-Polo, C.; del Mar Cledera-Castro, M.; Moratilla Soria, B.Y. Reviewing the anaerobic digestion of food waste: From waste generation and anaerobic processes to its perspectives. Appl. Sci. 2018, 8, 1804. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Wang, W.; Xue, L.; Chen, C.; Liu, G.; Zhang, R. Effects of ammonia on the anaerobic digestion of food waste: Process performance and microbial community. Energy Fuels 2016, 30, 5749–5757. [Google Scholar] [CrossRef]
- Lu, H.; Oehmen, A.; Virdis, B.; Keller, J.; Yuan, Z. Obtaining highly enriched cultures of Candidatus Accumulibacter phosphates through alternating carbon sources. Water Res. 2006, 40, 3838–3848. [Google Scholar] [CrossRef]
- Camejo, P.Y.; Owen, B.R.; Martirano, J.; Ma, J.; Kapoor, V.; Santo Domingo, J.; McMahon, K.D.; Noguera, D.R. Candidatus Accumulibacter phosphatis clades enriched under cyclic anaerobic and microaerobic conditions simultaneously use different electron acceptors. Water Res. 2016, 102, 125–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oyserman, B.O.; Noguera, D.R.; Del Rio, T.G.; Tringe, S.G.; McMahon, K.D. Metatranscriptomic insights on gene expression and regulatory controls in Candidatus Accumulibacter phosphatis. ISME J. 2016, 10, 810–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, W.; Shin, S.G.; Han, G.; Cho, K.; Hwang, S. Structures of microbial communities found in anaerobic batch runs that produce methane from propionic acid--seeded from full-scale anaerobic digesters above a certain threshold. J. Biotechnol. 2015, 214, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, R.; O’Connor, S.M.; Chan, E.; Coates, J.D. Anaerobic degradation of benzene, toluene, ethylbenzene, and xylene compounds by Dechloromonas strain RCB. Appl. Environ. Microbiol. 2005, 71, 8649–8655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horn, M.A.; Ihssen, J.; Matthies, C.; Schramm, A.; Acker, G.; Drake, H.L. Dechloromonas denitrificans sp. nov., Flavobacterium denitrificans sp. nov., Paenibacillus anaericanus sp. nov., and Paenibacillus terrae strain MH72, and N2O-producing bacteria isolated from the gut of the earthworm Aporrectodea caliginosa. Int. J. Syst. Evol. Microbiol. 2005, 55, 1255–1265. [Google Scholar] [CrossRef]
- Breitenstein, A.; Wiegel, J.; Haertig, C.; Weiss, N.; Andreesen, J.R.; Lechner, U. Reclassification of Clostridium hydroxybenzoicum as Sedimentibacter hydroxybenzoicus gen. nov., comb. nov., and description of Sedimentibacter saalensis sp. nov. Int. J. Syst. Evol. Microbiol. 2002, 52, 801–807. [Google Scholar]
- Gong, W.; Xie, B.; Deng, S.; Fan, Y.; Tang, X.; Liang, H. Enhancement of anaerobic digestion effluent treatment by microalgae immobilization: Characterized by fluorescence excitation-emission matrix coupled with parallel factor analysis in the photobioreactor. Sci. Total Environ. 2019, 678, 105–113. [Google Scholar] [CrossRef]
- Keating, C.; Chin, J.P.; Hughes, D.; Manesiotis, P.; Cysneiros, D.; Mahony, T.; Smith, C.J.; McGrath, J.W.; O’Flaherty, V. Biological phosphorus removal during high-rate, low-temperature, anaerobic digestion of wastewater. Front. Microbiol. 2016, 7, 226. [Google Scholar] [CrossRef] [Green Version]
- Hemp, J.; Ward, L.M.; Pace, L.A.; Fischer, W.W. Draft genome sequence of Levilinea saccharolytica KIBI-1, a member of the Chloroflexi class Anaerolineae. Genome Announc. 2015, 3, e01357-15. [Google Scholar] [CrossRef] [Green Version]
- Yamada, T.; Sekiguchi, Y.; Hanada, S.; Imachi, H.; Ohashi, A.; Harada, H.; Kamagata, Y. Anaerolinea thermolimosa sp. nov., Levilinea saccharolytica gen. nov., sp. nov. and Leptolinea tardivitalis gen. nov., sp. nov., novel filamentous anaerobes, and description of the new classes Anaerolineae classis nov. and Caldilineae classis nov. in the bacterial phylum Chloroflexi. Int. J. Syst. Evol. Microbiol. 2006, 56, 1331–1340. [Google Scholar]
- Wang, S.; Hou, X.; Su, H. Exploration of the relationship between biogas production and microbial community under high salinity conditions. Sci. Rep. 2017, 7, 1149. [Google Scholar]
- Takai, K.; Abe, M.; Miyazaki, M.; Koide, O.; Nunoura, T.; Imachi, H.; Inagaki, F.; Kobayashi, T. Sunxiuqinia faeciviva sp. nov., a facultatively anaerobic organoheterotroph of the Bacteroidetes isolated from deep subseafloor sediment. Int. J. Syst. Evol. Microbiol. 2013, 63, 1602–1609. [Google Scholar] [CrossRef] [PubMed]
- Takai, K.; Horikoshi, K. Thermosipho japonicus sp. nov., an extremely thermophilic bacterium isolated from a deep-sea hydrothermal vent in Japan. Extremophiles 2000, 4, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Nesbø, C.L.; Bradnan, D.M.; Adebusuyi, A.; Dlutek, M.; Petrus, A.K.; Foght, J.; Doolittle, W.F.; Noll, K.M. Mesotoga prima gen. nov., sp. nov., the first described mesophilic species of Thermotogales. Extremophiles 2012, 16, 387–393. [Google Scholar] [CrossRef]
- Ueki, A.; Akasaka, H.; Suzuki, D.; Ueki, K. Paludibacter propionicigenes gen. nov., sp. nov., a novel strictly anaerobic, gram-negative, propionate-producing bacterium isolated from plant residue in irrigated rice-field soil in Japan. Int. J. Syst. Evol. Microbiol. 2006, 56, 39–44. [Google Scholar] [CrossRef] [Green Version]
- Singleton, D.R.; Lee, J.; Dickey, A.N.; Stroud, A.; Scholl, E.H.; Wright, F.A.; Aitken, M.D. Polyphasic characterization of four soil-derived phenanthrene-degrading Acidovorax strains and the proposal of Acidovorax carolinensis sp. nov. Syst. Appl. Microbiol. 2018, 41, 460–472. [Google Scholar]
- Sun, W.; Li, Y.; McGuinness, L.R.; Luo, S.; Huang, W.; Kerkhof, L.J.; Mack, E.E.; Häggblom, M.M.; Fennell, D.E. Identification of anaerobic aniline-degrading bacteria at a contaminated industrial site. Environ. Sci. Technol. 2015, 49, 11079–11088. [Google Scholar] [CrossRef]
- Zamanzadeh, M.; Hagen, L.H.; Svensson, K.; Linjordet, R.; Horn, S.J. Anaerobic digestion of food waste: Effect of recirculation and temperature on performance and microbiology. Water Res. 2016, 96, 246–254. [Google Scholar] [CrossRef]
- Lyu, Z.; Shao, N.; Akinyemi, T.; Whitman, W.B. Methanogenesis. Curr. Biol. 2018, 28, R727–R732. [Google Scholar] [CrossRef] [Green Version]
- Musa, M.A.; Idrus, S. Effect of Hydraulic Retention Time on the Treatment of Real Cattle Slaughterhouse Wastewater and Biogas Production from HUASB Reactor. Water 2020, 12, 490. [Google Scholar] [CrossRef] [Green Version]




| Plants | Feedstock Mixing Ratio (%:%) | Reactor Volume (1000 m3) | f HRT (day) | Influent | Effluent | CH4 Yield (m3 CH4/kg VS) | pH | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Feedstock Concentration (g COD/L) | g C/N | h OLR (kg VS/m3/d) | Nutrient (%) | i VFA (g/L) | j TAN (g/L) | T-Alkalinity (g CaCO3/L) | ||||||||
| Protein | Fat | Carbohydrate | ||||||||||||
| A_FD | a FW:b S (94:6) | 28 | 19 | 18 ± 1.1 | 8.2 ± 0.2 | 1.5 ± 0.0 | 44.7 | 23.3 | 32.0 | 0.21 ± 0.0 | 1.3 ± 0.1 | 3.8 ± 0.2 | 0.3 ± 0.0 | 8.0 ± 0.1 |
| B_FD | c FWL:S (72:28) | 39 | 14.8 | 38 ± 0.5 | 8.0 ± 0.1 | 2.6 ± 0.1 | 46.2 | 31.6 | 22.2 | 0.28 ± 0.0 | 1.1 ± 0.0 | 3.7 ± 0.1 | 0.24 ± 0.1 | 7.9 ± 0.1 |
| C_FD | FWL:S (83:17) | 75 | 22 | 36 ± 0.6 | 10.1 ± 1.0 | 0.9 ± 0.0 | 45.2 | 41.0 | 13.9 | 0.27 ± 0.1 | 2.0 ± 0.2 | 5.1 ± 0.2 | 0.41 ± 0.1 | 8.0 ± 0.1 |
| D_FD | FWL:S (96:4) | 180 | 39 | 54 ± 0.1 | 7.0 ± 0.1 | 0.8 ± 0.1 | 55.7 | 35.1 | 9.2 | 0.54 ± 0.1 | 2.5 ± 0.1 | 5.0 ± 0.3 | 0.30 ± 0.2 | 8.2 ± 0.2 |
| E_SD | d PS:e SE (11:89) | 17.5 | 17.3 | 22 ± 0.1 | 6.4 ± 0.6 | 1.6 ± 0.1 | 58.2 | 26.9 | 14.9 | 0.80 ± 0.1 | 0.8 ± 0.0 | 2.5 ± 0.0 | 0.28 ± 0.1 | 7.9 ± 0.1 |
| F_SD | PS:SE (59:41) | 21 | 28 | 49 ± 1.8 | 7.3 ± 0.5 | 1.4 ± 0.0 | 44.3 | 13.6 | 42.1 | 0.32 ± 0.0 | 1.1 ± 0.0 | 3.1 ± 0.0 | 0.14 ± 0.1 | 7.9 ± 0.0 |
| G_SD | PS:SE (47:35:18) | 25.12 | 36 | 18 ± 1.1 | 6.1 ± 0.1 | 0.6 ± 0.0 | 66.4 | 10.5 | 23.1 | 0.25 ± 0.0 | 0.8 ± 0.0 | 2.2 ± 0.1 | 0.24 ± 0.0 | 7.6 ± 0.1 |
| Genus | Metabolism |
|---|---|
| Stygiolobus | chemolithotrophic (S0+H2) |
| Methanomassiliicoccus | methylotrophic |
| Methanococcus | hydrogenotrophic |
| Methanothermobacter | hydrogenotrophic |
| Methanoculleus | hydrogenotrophic |
| Methanosaeta | acetoclastic |
| Methanosarcina | acetoclastic and hydrogenotrophic |
| Methanomethylovorans | methylotrophic |
| Methanococcoides | methylotrophic |
| Methanosalsum | methylotrophic |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sposob, M.; Moon, H.-S.; Lee, D.; Yun, Y.-M. Microbiome of Seven Full-Scale Anaerobic Digestion Plants in South Korea: Effect of Feedstock and Operational Parameters. Energies 2021, 14, 665. https://doi.org/10.3390/en14030665
Sposob M, Moon H-S, Lee D, Yun Y-M. Microbiome of Seven Full-Scale Anaerobic Digestion Plants in South Korea: Effect of Feedstock and Operational Parameters. Energies. 2021; 14(3):665. https://doi.org/10.3390/en14030665
Chicago/Turabian StyleSposob, Michal, Hee-Sung Moon, Dongjin Lee, and Yeo-Myeong Yun. 2021. "Microbiome of Seven Full-Scale Anaerobic Digestion Plants in South Korea: Effect of Feedstock and Operational Parameters" Energies 14, no. 3: 665. https://doi.org/10.3390/en14030665
APA StyleSposob, M., Moon, H. -S., Lee, D., & Yun, Y. -M. (2021). Microbiome of Seven Full-Scale Anaerobic Digestion Plants in South Korea: Effect of Feedstock and Operational Parameters. Energies, 14(3), 665. https://doi.org/10.3390/en14030665