Purification of Methyl Acetate/Water Mixtures from Chemical Interesterification of Vegetable Oils by Pervaporation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
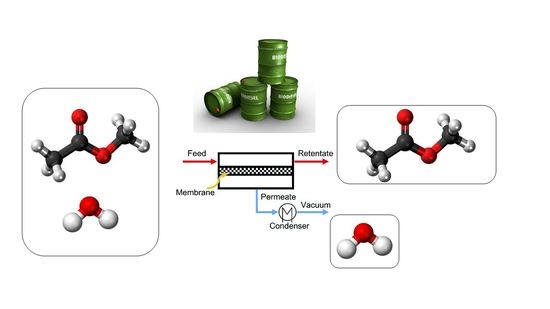
2.2. Pervaporation Setup
2.3. Experimental Procedure
2.4. Analytical Methods
3. Results
3.1. Modelling of Experimental Results
- (1)
- Transport from the feed fluid to the surface of the membrane.
- (2)
- Solution of the component in the membrane.
- (3)
- Diffusion of the component through the membrane.
- (4)
- Desorption of the component in the permeate phase.
- (5)
- Transport from the surface of the membrane to the vapor phase.
3.2. Pervaporation Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Casas, A.; Ramos, M.J.; Pérez, Á. New trends in biodiesel production: Chemical interesterification of sunflower oil with methyl acetate. Biomass Bioenergy 2011, 35, 1702–1709. [Google Scholar] [CrossRef]
- Casas, A.; Ramos, M.J.; Pérez, T. Kinetics of chemical interesterification of sunflower oil with methyl acetate for biodiesel and triacetin production. Chem. Eng. J. 2011, 171. [Google Scholar] [CrossRef]
- Wong, W.Y.; Lim, S.; Pang, Y.L.; Shuit, S.H.; Chen, W.H.; Lee, K.T. Synthesis of renewable heterogeneous acid catalyst from oil palm empty fruit bunch for glycerol-free biodiesel production. Sci. Total Environ. 2020, 727. [Google Scholar] [CrossRef]
- Brondani, L.N.; Ribeiro, J.S.; Castilhos, F. A new kinetic model for simultaneous interesterification and esterification reactions from methyl acetate and highly acidic oil. Renew. Energy 2020, 156, 579–590. [Google Scholar] [CrossRef]
- Kampars, V.; Abelniece, Z.; Lazdovica, K.; Kampare, R. Interesterification of rapeseed oil with methyl acetate in the presence of potassium tert-butoxide solution in tetrahydrofuran. Renew. Energy 2020, 158, 668–674. [Google Scholar] [CrossRef]
- Xu, Y.; Du, W.; Liu, D.; Zeng, J. A novel enzymatic route for biodiesel production from renewable oils in a solvent-free medium. Biotechnol. Lett. 2003, 25, 1239–1241. [Google Scholar] [CrossRef]
- Du, W.; Xu, Y.; Liu, D.; Zeng, J. Comparative study on lipase-catalyzed transformation of soybean oil for biodiesel production with different acyl acceptors. J. Mol. Catal. B Enzym. 2004, 30, 125–129. [Google Scholar] [CrossRef]
- Ognjanovic, N.; Bezbradica, D.; Knezevic-Jugovic, Z. Enzymatic conversion of sunflower oil to biodiesel in a solvent-free system: Process optimization and the immobilized system stability. Bioresour. Technol. 2009, 100, 5146–5154. [Google Scholar] [CrossRef]
- Orçaire, O.; Buisson, P.; Pierre, A.C. Application of silica aerogel encapsulated lipases in the synthesis of biodiesel by transesterification reactions. J. Mol. Catal. B Enzym. 2006, 42, 106–113. [Google Scholar] [CrossRef]
- Xu, Y.; Du, W.; Liu, D. Study on the kinetics of enzymatic interesterification of triglycerides for biodiesel production with methyl acetate as the acyl acceptor. J. Mol. Catal. B Enzym. 2005, 32, 241–245. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Liang, S.H.; Chen, S.S.; Su, C.H.; Lin, J.H.; Chien, C.C. Enzymatic production of biodiesel from insect fat using methyl acetate as an acyl acceptor: Optimization by using response surface methodology. Energy Convers. Manag. 2018, 158, 168–175. [Google Scholar] [CrossRef]
- Tavares, G.R.; Gonçalves, J.E.; dos Santos, W.D.; da Silva, C. Enzymatic interesterification of crambe oil assisted by ultrasound. Ind. Crop. Prod. 2017, 97, 218–223. [Google Scholar] [CrossRef]
- Subhedar, P.B.; Gogate, P.R. Ultrasound assisted intensification of biodiesel production using enzymatic interesterification. Ultrason. Sonochemistry 2016, 29, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Saka, S.; Isayama, Y. A new process for catalyst-free production of biodiesel using supercritical methyl acetate. Fuel 2009, 88, 1307–1313. [Google Scholar] [CrossRef] [Green Version]
- Goembira, F.; Saka, S. Factors affecting biodiesel yield in interesterification of rapeseed oil by supercritical Methyl Acetate. Green Energy Technol. 2012, 108, 147–152. [Google Scholar] [CrossRef]
- Sakdasri, W.; Ngamprasertsith, S.; Daengsanun, S.; Sawangkeaw, R. Lipid-based biofuel synthesized from palm-olein oil by supercritical ethyl acetate in fixed-bed reactor. Energy Convers. Manag. 2019, 182, 215–223. [Google Scholar] [CrossRef]
- Goembira, F.; Saka, S. Advanced supercritical Methyl acetate method for biodiesel production from Pongamia pinnata oil. Renew. Energy 2015, 83, 1245–1249. [Google Scholar] [CrossRef]
- Loehe, J.R.; van Ness, H.C.; Abbott, M.M. Vapor/Liquid/Liquid Equilibrium. Total-Pressure Data and GE for Water/Methyl Acetate at 50C. J. Chem. Eng. Data. 1983, 28, 405–407. [Google Scholar] [CrossRef]
- Jyoti, G.; Keshav, A.; Anandkumar, J. Review on Pervaporation: Theory, Membrane Performance, and Application to Intensification of Esterification Reaction. J. Eng. 2015, 2015, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Ho, W.S.W.; Sirkar, K.K. Membrane Handbook, 1st ed.; Kluwer Academic Publishers: New York, NY, USA, 1992; p. 954. [Google Scholar]
- Borisov, I.L.; Kujawska, A.; Knozowska, K.; Volkov, V.V.; Kujawski, W. Influence of feed flow rate, temperature and feed concentration on concentration polarization effects during separation of water-methyl acetate solutions with high permeable hydrophobic pervaporation PDMS membrane. J. Membr. Sci. 2018, 564, 1–9. [Google Scholar] [CrossRef]
- Seider, W.D.; Seader, J.D.; Lewin, D.R. Process Design Principles: Synthesis, Analysis & Evaluation; Wiley: Hoboken, NJ, USA, 1998; 1122p. [Google Scholar]
- Casas, A.; Ramos, M.J.; Pérez, T. Adsorption equilibrium and kinetics of methyl acetate/methanol and methyl acetate/water mixtures on zeolite 5A. Chem. Eng. J. 2013, 220. [Google Scholar] [CrossRef]
- Okubo, A. Diffusion: Mass Transfer in Fluid Systems. E. L. Cussler. Q. Rev. Biol. 1987, 62, 131. [Google Scholar] [CrossRef]
- Sitaraman, R.; Ibrahim, S.H.; Kuloor, N.R. A Generalized Equation for Diffusion in Liquids. J. Chem. Eng. Data 2002, 8, 198–201. [Google Scholar] [CrossRef]
- Hsieh, C.-T.; Ji, W.-Y.; Lin, H.-M.; Lee, M.-J. Multiphase equilibria for mixtures containing water, acetic acid, propionic acid, methyl acetate and methyl propionate. Fluid Phase Equilibria 2008, 271, 69–75. [Google Scholar] [CrossRef]
- Tian, X.; Jiang, X.; Zhu, B.; Xu, Y. Effect of the permeants/polymer interactions on the pervaporative separation properties of the P(VDF-co-HFP) membrane. Eur. Polym. J. 2006, 42, 3041–3049. [Google Scholar] [CrossRef]






| Temperature (°C) | Water (wt. %) | 1/Qg,w (kg h−1 m−2 Pa−1) | 1/Qbl,w (kg h−1 m−2 Pa−1) | 1/Qm,w (kg h−1 m−2 Pa−1) |
|---|---|---|---|---|
| 50 | 2.2 | 173,239 | 84 | 173,155 |
| 50 | 6.6 | 21,220 | 61 | 21,159 |
| 70 | 2.1 | 172,808 | 125 | 172,684 |
| 70 | 7.0 | 27,535 | 148 | 27,387 |
| Parameter | Value |
|---|---|
| Daoo/(Aal) | 15.01 |
| Ea (kJ mol−1) | 52.05 |
| AaKa | 4.38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casas, A.; Pérez, Á.; Ramos, M.J. Purification of Methyl Acetate/Water Mixtures from Chemical Interesterification of Vegetable Oils by Pervaporation. Energies 2021, 14, 775. https://doi.org/10.3390/en14030775
Casas A, Pérez Á, Ramos MJ. Purification of Methyl Acetate/Water Mixtures from Chemical Interesterification of Vegetable Oils by Pervaporation. Energies. 2021; 14(3):775. https://doi.org/10.3390/en14030775
Chicago/Turabian StyleCasas, Abraham, Ángel Pérez, and María Jesús Ramos. 2021. "Purification of Methyl Acetate/Water Mixtures from Chemical Interesterification of Vegetable Oils by Pervaporation" Energies 14, no. 3: 775. https://doi.org/10.3390/en14030775
APA StyleCasas, A., Pérez, Á., & Ramos, M. J. (2021). Purification of Methyl Acetate/Water Mixtures from Chemical Interesterification of Vegetable Oils by Pervaporation. Energies, 14(3), 775. https://doi.org/10.3390/en14030775