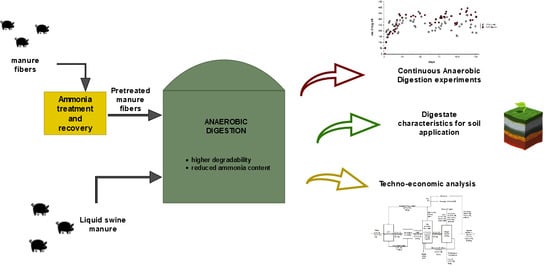
Improving the Anaerobic Digestion of Swine Manure through an Optimized Ammonia Treatment: Process Performance, Digestate and Techno-Economic Aspects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Feedstock and Inoculum
2.2. AAS Pretreatment
2.3. Experimental Set Up of Continuous AD
2.4. Analytical Methods and Compositional Analysis
2.5. Theoretical Calculations and Assumptions
2.6. Techno-Economic Analysis
3. Results and Discussion
3.1. Process Characteristics, Productivity, and Stability
3.2. Reduction Efficiency of Major Organic Components and Digestate Quality
3.3. Preliminary Techno-Economic Analysis of AD Coupled to AAS and NH3 Recovery
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holm-Nielsen, J.B.; Al Seadi, T.; Oleskowicz-Popiel, P. The future of anaerobic digestion and biogas utilization. Bioresour. Technol. 2009, 100, 5478–5484. [Google Scholar] [CrossRef] [PubMed]
- Asam, Z.; Poulsen, T.G.; Nizami, A.; Rafique, R.; Kiely, G.; Murphy, J.D. How can we improve biomethane production per unit of feedstock in biogas plants? Appl. Energy 2011, 88, 2013–2018. [Google Scholar] [CrossRef]
- Hansen, K.H.; Angelidaki, I.; Ahring, B.K. Anaerobic digestion of swine manure: Inhibition by ammonia. Water Res. 1998, 32, 5–12. [Google Scholar] [CrossRef]
- Hamelin, L.; Wesnæs, M.; Wenzel, H.; Petersen, B.M. Environmental Consequences of Future Biogas Technologies Based on Separated Slurry. Environ. Sci. Technol. 2011, 45, 5869–5877. [Google Scholar] [CrossRef] [PubMed]
- Weiland, P. Biogas production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2010, 85, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Jurado, E.; Skiadas, I.V.; Gavala, H.N. Enhanced methane productivity from manure fibers by aqueous ammonia soaking pretreatment. Appl. Energy 2013, 109, 104–111. [Google Scholar] [CrossRef]
- Foged, H.L.; Flotats, X.; Bonmati Blasi, A.; Palatsi, J.; Magri, A.; Schelde, K.M. Inventory of Manure Process Activities in Europe; Technical Report No. I concerning “Manure Processing Activities in Europe” to the European Comission; Directorate General Environment; Agro Business Park A/S: Tjele, Denmark, 2011. [Google Scholar]
- Thygesen, O.; Triolo, J.M.; Sommer, S.G. Anaerobic digestion of pig manure fibres from commercial pig slurry separation units. Biosyst. Eng. 2014, 123, 91–96. [Google Scholar] [CrossRef]
- Jurado, E.; Gavala, H.N.; Skiadas, I.V. Enhancement of methane yield from wheat straw, miscanthus and willow using aqueous ammonia soaking. Environ. Technol. 2013, 34, 2069–2075. [Google Scholar] [CrossRef]
- Antonopoulou, G.; Gavala, H.N.; Skiadas, I.V.; Lyberatos, G. The Effect of Aqueous Ammonia Soaking Pretreatment on Methane Generation Using Different Lignocellulosic Biomasses. Waste Biomass Valori 2015, 6, 281–291. [Google Scholar] [CrossRef] [Green Version]
- Lymperatou, A.; Gavala, H.N.; Skiadas, I.V. Aqueous Ammonia Soaking of Wheat Straw at Ambient Temperature for Enhancing the Methane Yield: Process Optimization by Response Surface Methodology. Waste Biomass Valori 2020, 11, 4821–4835. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, Y.Y.; Kim, T.H. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour. Technol. 2016, 199, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Lymperatou, A.; Gavala, H.N.; Esbensen, K.H.; Skiadas, I.V. AMMONOX: Ammonia for Enhancing Biogas Yield and Reducing NOx—Analysis of Effects of Aqueous Ammonia Soaking on Manure Fibers. Waste Biomass Valori 2015, 6, 449–457. [Google Scholar] [CrossRef] [Green Version]
- Lymperatou, A.; Gavala, H.N.; Skiadas, I.V. Optimization of Aqueous Ammonia Soaking of manure fibers by Response Surface Methodology for unlocking the methane potential of swine manure. Bioresour. Technol. 2017, 244, 509–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrère, H.; Antonopoulou, G.; Affes, R.; Passos, F.; Battimelli, A.; Lyberatos, G.; Ferrer, I. Review of feedstock pretreatment strategies for improved anaerobic digestion: From lab-scale research to full-scale application. Bioresour. Technol. 2016, 199, 386–397. [Google Scholar] [CrossRef]
- Cestonaro do Amaral, A.; Kunz, A.; Radis Steinmetz, R.L.; Scussiato, L.A.; Tápparo, D.C.; Gaspareto, T.C. Influence of solid-liquid separation strategy on biogas yield from a stratified swine production system. J. Environ. Manag. 2016, 168, 229–235. [Google Scholar] [CrossRef] [Green Version]
- Jurado, E.; Antonopoulou, G.; Lyberatos, G.; Gavala, H.N.; Skiadas, I.V. Continuous anaerobic digestion of swine manure: ADM1-based modelling and effect of addition of swine manure fibers pretreated with aqueous ammonia soaking. Appl. Energy 2016, 172, 190–198. [Google Scholar] [CrossRef] [Green Version]
- APHA. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association/American Water Works Association/Water Pollution Control Federation, Ed.; American Public Health Association/American Water Works Association/Water Pollution Control Federation: Washington, DC, USA, 2005. [Google Scholar]
- Edwards, G.P.; Molof, A.H.; Schneeman, R.W. Determination of Orthophosphate in Fresh and Saline Waters. Am. Water Work. Assoc. 1965, 57, 917–925. [Google Scholar] [CrossRef]
- Lymperatou, A.; Skiadas, I.V.; Gavala, H.N. Anaerobic co-digestion of swine manure and crude glycerol derived from animal fat—Effect of hydraulic retention time. AIMS Environ. Sci. 2018, 5, 105–116. [Google Scholar] [CrossRef]
- Galí, A.; Benabdallah, T.; Astals, S.; Mata-Alvarez, J. Modified version of ADM1 model for agro-waste application. Bioresour. Technol. 2009, 100, 2783–2790. [Google Scholar] [CrossRef]
- Symons, G.E.; Buswell, A.M. The methane fermentation of carbohydrates. J. Am. Chem. Soc. 1933, 55, 2028–2036. [Google Scholar] [CrossRef]
- Møller, H.B.; Sommer, S.G.; Ahring, B.K. Methane productivity of manure, straw and solid fractions of manure. Biomass Bioenergy 2004, 26, 485–495. [Google Scholar] [CrossRef]
- Batstone, D.; Rodriguez, J. Modelling anaerobic digestion processes. In Anaerobic Biotechnology; Fang, H., Zhang, T., Eds.; Imperial College Press: London, UK, 2015; pp. 133–160. [Google Scholar]
- Romero-Güiza, M.S.; Astals, S.; Chimenos, J.M.; Martínez, M.; Mata-Alvarez, J. Improving anaerobic digestion of pig manure by adding in the same reactor a stabilizing agent formulated with low-grade magnesium oxide. Biomass Bioenergy 2014, 67, 243–251. [Google Scholar] [CrossRef]
- Bonmati, A.; Flotats, X.; Mateu, L.; Campos, E. Study of thermal hydrolysis as a pretreatment to mesophilic anaerobic digestion of pig slurry. Water Sci. Technol. 2001, 44, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Duan, N.; Zhang, D.; Lin, C.; Zhang, Y.; Zhao, L.; Liu, H.; Liu, Z. Effect of organic loading rate on anaerobic digestion of pig manure: Methane production, mass flow, reactor scale and heating scenarios. J. Environ. Manage. 2019. [Google Scholar] [CrossRef]
- Møller, H.B.; Nielsen, A.M.; Nakakubo, R.; Olsen, H.J. Process performance of biogas digesters incorporating pre-separated manure. Livest. Sci. 2007, 112, 217–223. [Google Scholar] [CrossRef]
- Vergote, T.L.I.; De Dobbelaere, A.E.J.; Willems, B.; Leenknegt, J.; Buysse, J.; Volcke, E.I.P.; Meers, E. Stability of Thermophilic Pig Manure Mono-digestion: Effect of Thermal Pre-treatment and Separation. Front. Energy Res. 2020, 8, 1–15. [Google Scholar] [CrossRef]
- Sawatdeenarunat, C.; Surendra, K.C.; Takara, D.; Oechsner, H.; Khanal, S.K. Anaerobic digestion of lignocellulosic biomass: Challenges and opportunities. Bioresour. Technol. 2015, 178, 178–186. [Google Scholar] [CrossRef]
- Chen, J.L.; Ortiz, R.; Steele, T.W.J.; Stuckey, D.C. Toxicants inhibiting anaerobic digestion: A review. Biotechnol. Adv. 2014, 32, 1523–1534. [Google Scholar] [CrossRef]
- Capson-Tojo, G.; Moscoviz, R.; Astals, S.; Robles, A.; Steyer, J.P. Unraveling the literature chaos around free ammonia inhibition in anaerobic digestion. Renew. Sustain. Energy Rev. 2020, 117, 109487. [Google Scholar] [CrossRef]
- Yenigün, O.; Demirel, B. Ammonia inhibition in anaerobic digestion: A review. Process Biochem. 2013, 48, 901–911. [Google Scholar] [CrossRef]
- Fotidis, I.A.; Karakashev, D.; Angelidaki, I. The dominant acetate degradation pathway/methanogenic composition in full-scale anaerobic digesters operating under different ammonia levels. Int. J. Environ. Sci. Technol. 2014, 11, 2087–2094. [Google Scholar] [CrossRef] [Green Version]
- Rajagopal, R.; Massé, D.I.; Singh, G. A critical review on inhibition of anaerobic digestion process by excess ammonia. Bioresour. Technol. 2013, 143, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Møller, H.B.; Sommer, S.G.; Ahring, B.K. Separation efficiency and particle size distribution in relation to manure type and storage conditions. Bioresour. Technol. 2002, 85, 189–196. [Google Scholar] [CrossRef]
- Møller, H.B.; Hansen, J.D.; Sørensen, C.A.G. Nutrient Recovery by Solid-Liquid Separation and Methane Productivity of Solids. Trans. ASABE 2007, 50, 193–200. [Google Scholar] [CrossRef]
- Jurado, E.; Gavala, H.N.; Skiadas, I.V. Application of aqueous ammonia soaking for enhancement of methane potential of swine manure. In Proceedings of the 4th International Conference on Engineering for Waste and Biomass Valorisation, Porto, Portugal, 10–13 September 2012; pp. 389–394. [Google Scholar]
- Monlau, F.; Sambusiti, C.; Barakat, A.; Guo, X.M.; Latrille, E.; Trably, E.; Steyer, J.-P.; Carrere, H. Predictive Models of Biohydrogen and Biomethane Production Based on the Compositional and Structural Features of Lignocellulosic Materials. Environ. Sci. Technol. 2012, 46, 12217–12225. [Google Scholar] [CrossRef]
- Triolo, J.M.; Sommer, S.G.; Møller, H.B.; Weisbjerg, M.R.; Jiang, X.Y. A new algorithm to characterize biodegradability of biomass during anaerobic digestion: Influence of lignin concentration on methane production potential. Bioresour. Technol. 2011, 102, 9395–9402. [Google Scholar] [CrossRef]
- Barakat, A.; Monlau, F.; Steyer, J.P.; Carrère, H. Effect of lignin-derived and furan compounds found in lignocellulosic hydrolysates on biomethane production. Bioresour. Technol. 2012, 104, 90–99. [Google Scholar] [CrossRef]
- Monlau, F.; Sambusiti, C.; Ficara, E.; Aboulkas, A.; Barakat, A.; Carrere, H. New opportunities for agricultural digestate valorization: Current situation and perspectives. Energy Environ. Sci. 2015, 8, 2600–2621. [Google Scholar] [CrossRef]
- Teglia, C.; Tremier, A.; Martel, J.-L. Characterization of Solid Digestates: Part 1, Review of Existing Indicators to Assess Solid Digestates Agricultural Use. Waste Biomass Valori 2011, 2, 43–58. [Google Scholar] [CrossRef]
- Nkoa, R. Agricultural benefits and environmental risks of soil fertilization with anaerobic digestates: A review. Agron. Sustain. Dev. 2014, 34, 473–492. [Google Scholar] [CrossRef] [Green Version]
- Bernal, M.P.; Sanchez-Monedero, M.A.; Paredes, C.; Roig, A. Carbon mineralization from organic wastes at different stages during the incubation in soil. Agric. Ecosyst. Environ. 1998, 69, 175–189. [Google Scholar] [CrossRef]
- Akhiar, A.; Battimelli, A.; Torrijos, M.; Carrere, H. Comprehensive characterization of the liquid fraction of digestates from full-scale anaerobic co-digestion. Waste Manag. 2017, 59, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Bolzonella, D.; Fatone, F.; Gottardo, M.; Frison, N. Nutrients recovery from anaerobic digestate of agro-waste: Techno-economic assessment of full scale applications. J. Environ. Manage. 2018, 216, 111–119. [Google Scholar] [CrossRef] [PubMed]
- DEA, Danish Energy Agency. Samfundsøkonomiske Beregningsforudsætninger for Energipriser og Emissioner, Oktober 2019. Available online: https://ens.dk/sites/ens.dk/files/Analyser/samfundsoekonomiske_beregningsforudsaetninger_for_energipriser_og_emissioner_2019.pdf (accessed on 9 December 2020).
- DEA, Danish Energy Agency. Pristillæg-biogas-2020. Available online: https://ens.dk/sites/ens.dk/files/Stoette_vedvarende_energi/pristillaeg-biogas-2020.pdf (accessed on 9 December 2020).
- Frandsen, T.Q.; Rodhe, L.; Baky, A.; Edstrom, M.; Sipila, I.; Petersen, S.L.; Tybirk, K. Best Available Technologies for Pig Manure Biogas Plants in the Baltic Sea Region; Baltic Sea 2020: Stockholm, Sweden, 2011. [Google Scholar]
- Pöschl, M.; Ward, S.; Owende, P. Evaluation of energy efficiency of various biogas production and utilization pathways. Appl. Energy 2010, 87, 3305–3321. [Google Scholar] [CrossRef]


| Component | Swine Manure | Non-Pretreated Manure Fibers 2 | AAS-Treated Manure Fibers 2 |
|---|---|---|---|
| TS (% wet mass) | 2.2 | 3.2 1 | 3.1 |
| VS (% wet mass) | 1.5 | 2.2 | 2.2 |
| Cellulose (% TS) | 12.3 | 30.4 | 31.2 |
| Hemicellulose (% TS) | 9.2 | 21.7 | 16.1 |
| Proteins (% TS) | 22.9 | 15.1 | 19.9 |
| Lipids (% TS) | 7.7 | 7.8 | 5.9 |
| Lignin (% TS) | 15.8 | 16.6 | 16.7 |
| TAN (% TS) | 1.09 | 0.37 | 0.99 |
| Characteristic | NP Digester | AAS Digester | Reference Digester |
|---|---|---|---|
| Feed ratio g TS manure: g TS fibers | 2:1 | 2:1 | 1:0 |
| C/Norg of influent | 11.1 | 10.2 | 9.4 |
| Organic Loading Rate (g VS/L/d) | 1.1 ± 0.2 | 1.0 ± 0.2 | 0.9 ± 0.1 |
| Hydraulic Retention Time (d) | 18.2 ± 1.1 | 17.9 ± 1.3 | 17.6 ± 0.1 |
| Solid Retention Time (d) | 26.7 ± 1.1 | 25.9 ± 1.9 | 20.5 ± 0.3 |
| VFA concentration 1 (g/L) | 0.19 ± 0.07 | 0.26 ± 0.07 | 0.22 ± 0.01 |
| pH1 | 8.1 ± 0.0 | 8.1 ± 0.1 | 8.2 ± 0.3 |
| Soluble COD 1 (g/L) | 2.24 ± 0.07 | 2.34 ± 0.23 | 3.08 ± 1.34 |
| TAN concentration 1 (g/L) | 1.82 ± 0.26 | 2.04 ± 0.01 | 2.73 ± 0.05 |
| FAN concentration 1 (g/L) | 0.25 ± 0.03 | 0.29 ± 0.01 | 0.46 ± 0.01 |
| TS1 (g/L) inside the digester | 26.63 ± 0.95 | 22.17 ± 1.53 | 26.61 ± 1.00 |
| VS1 (g/L) inside the digester | 16.40 ± 0.69 | 13.24 ± 1.01 | 15.29 ± 0.83 |
| TSS1 (g/L) inside the digester | 20.35 ± 0.07 | 17.05 ± 0.07 | 16.70 ± 0.64 |
| Biogas productivity (L/L/d) | 0.41 ± 0.08 | 0.48± 0.06 | 0.43 ± 0.06 |
| Methane productivity (L/L/d) | 0.25 ± 0.04 | 0.30 ± 0.04 | 0.28 ± 0.04 |
| CH4 (% biogas) | 61.8 ± 0.9 | 64.1 ± 1.1 | 66.0 ± 3.0 |
| Methane yield (mL/g TSfed) | 156 ± 37 | 215 ± 40 | 204 ± 34 |
| Methane yield (mL/ g VSfed) | 222 ± 54 | 314 ± 61 | 330 ± 61 |
| NP Digester | AAS Digester | |||||
|---|---|---|---|---|---|---|
| Component | Influent (g/kg) | Effluent (g/kg) | % Reduction | Influent (g/kg) | Effluent (g/kg) | % Reduction |
| VS | 21.87 ± 0.53 | 11.9 ± 1.93 | 45.8 | 20.32 ± 1.96 | 10.02 ± 2.16 | 50.7 |
| Cellulose | 4.76 ± 0.00 1 | 2.74 ± 0.02 | 42.6 | 4.53 ± 0.11 1 | 1.80 ± 0.11 | 60.3 |
| Hemicellulose | 3.83 ± 0.01 1 | 1.76 ± 0.02 | 54.1 | 2.81 ± 0.01 1 | 0.98 ± 0.06 | 65.3 |
| Proteins | 6.21 ± 0.79 1 | 3.70 ± 0.03 | 40.5 | 6.25 ± 1.20 1 | 3.27 ± 0.03 | 47.7 |
| Lipids | 2.37 ± 0.18 1 | 1.52 ± 0.13 | 35.6 | 2.01 ± 0.01 1 | 1. 11 ± 0.19 | 44.6 |
| Lignin | 4.92 ± 0.36 1 | 3.03 ± 0.00 | 38.5 | 4.61 ± 0.35 1 | 2.39 ± 0.10 | 48.2 |
| VFAs | 6.52 ± 0.54 | 0.19 ± 0.07 | 97.1 | 6.20 ± 0.42 | 0.26 ± 0.07 | 95.8 |
| Quality Parameter | NP Effluent | AAS Effluent |
|---|---|---|
| TS (% wet mass) | 2.0 ± 0.3 | 1.8 ± 0.3 |
| Total COD (g/g TS) | 0.1 ± 0.0 | 0.2 ± 0.0 |
| VS (% TS) | 58.5 ± 0.7 | 56.8 ± 3.6 |
| pH | 7.9 ± 0.1 | 7.9 ± 0.1 |
| C/N | 8.9 ± 0.0 | 8.4 ± 0.1 |
| Cellulose/Lignin | 0.90 ± 0.0 | 0.75 ± 0.0 |
| NH4+ − N (% Total N) | 73.8 ± 3.2 | 77.3 ± 1.9 |
| NH4+ − N (% TS) | 7.6 ± 1.2 | 11.1 ± 1.9 |
| Soluble COD (% Total COD) | 72.7 | 63.2 |
| Soluble C/N ratio | 2.4 ± 0.2 | 2.7 ± 0.3 |
| Soluble total N (g/L) | 2.6 ± 0.1 | 2.9 ± 0.0 |
| Soluble NH4+ − N (g/L) | 1.7 ± 0.0 | 1.8 ± 0.1 |
| Soluble PO43−− P (g/L) | 0.1 ± 0.0 | 0.1 ± 0.0 |
| Soluble K+ − K (g/L) | 5.1 ± 0.2 | 5.2 ± 0.4 |
| Process Characteristics | Units | Values | Input/Output/Target |
|---|---|---|---|
| HRT | (days) | 20 | Input |
| Fibers/wet mass input | (kg) | 3 | Input |
| TS in received manure fibers | (% mass) | 30 | Input |
| NH3 in received manure fibers | (% mass) | 0.2 | Input |
| Fresh manure mass input | (kg) | 10 | Input |
| TS in fresh manure | (% mass) | 6 | Input |
| NH3 in fresh manure | (% mass) | 0.7 (0.4) 1 | Input |
| TS in mixture for NH3 extraction | (% mass) | <12 | Input |
| Vacuum distillation pressure | (bar abs) | 0.13 | Input |
| Assumed TS conversion biogas reactor | (% mass) | 25 | Input |
| Steam input (250 °C) | (kg) | 1.0 | Input |
| NH3 in AAS treatment | (% mass) | ca. 6 | Target |
| TS in AAS treatment | (% mass) | ca. 4 | Target |
| NH3 in mixture after extraction | (% mass) | <0.5 | Target |
| Internal heat exchange | (MJ) | 3.0 | Output |
| Energy for compressor | (kJ) | 485 | Output |
| NH3 extraction temperature | (°C) | 47 | Output |
| AAS pretreatment temperature | (°C) | 14 | Output |
| Calculated total TS conversion rate | (% mass) | 36 | Output |
| Biogas output energy with AAS | (MJ) | 10.70 | Output |
| Scenario | MJ Produced | DKK per kg Digested | % Increase of Revenue due to AAS |
|---|---|---|---|
| 1-Swine manure (10 kg) | 5.17 | 0.091 | +135 |
| 2-Swine manure (10 kg) + NP fibers (3 kg) | 7.04 | 0.096 | +72 |
| 3-Swine manure (10 kg) + AAS manure fibers (3 kg) | 10.70 | 0.145 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lymperatou, A.; Rasmussen, N.B.; Gavala, H.N.; Skiadas, I.V. Improving the Anaerobic Digestion of Swine Manure through an Optimized Ammonia Treatment: Process Performance, Digestate and Techno-Economic Aspects. Energies 2021, 14, 787. https://doi.org/10.3390/en14030787
Lymperatou A, Rasmussen NB, Gavala HN, Skiadas IV. Improving the Anaerobic Digestion of Swine Manure through an Optimized Ammonia Treatment: Process Performance, Digestate and Techno-Economic Aspects. Energies. 2021; 14(3):787. https://doi.org/10.3390/en14030787
Chicago/Turabian StyleLymperatou, Anna, Niels B. Rasmussen, Hariklia N. Gavala, and Ioannis V. Skiadas. 2021. "Improving the Anaerobic Digestion of Swine Manure through an Optimized Ammonia Treatment: Process Performance, Digestate and Techno-Economic Aspects" Energies 14, no. 3: 787. https://doi.org/10.3390/en14030787
APA StyleLymperatou, A., Rasmussen, N. B., Gavala, H. N., & Skiadas, I. V. (2021). Improving the Anaerobic Digestion of Swine Manure through an Optimized Ammonia Treatment: Process Performance, Digestate and Techno-Economic Aspects. Energies, 14(3), 787. https://doi.org/10.3390/en14030787