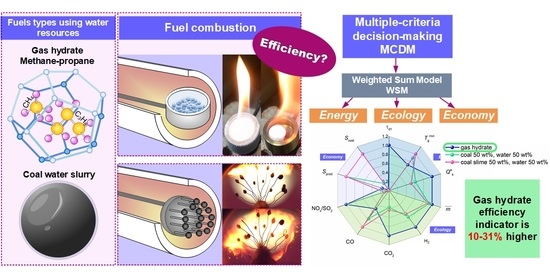
1.1. Gas Hydrates
Alternative energy sources are becoming increasingly attractive with the growing global energy consumption. This is especially true for gas hydrates [
1,
2]. These compounds of water and gas—most commonly methane (up to 90%)—are formed at low temperatures and high pressures [
3]. Hydrogen-bonded water molecules form a stable cage, and methane molecules are trapped within. In general, there are three main structures of gas hydrates: structural I (sI), structural II (sII), and structure H (sH) [
4]. In sI hydrate, water molecules are automatically arranged to form cavities with 5
12 cages and 5
126
2 cages [
4,
5]. Natural gas hydrates are concentrated in deep water areas, mostly on the seabed and ocean floor. Marine hydrates contain up to 90% of methane. The largest hydrate accumulations are located in the southeast and west of North America, near Canada, Peru, Costa Rica, South Korea, Mexico, and Japan. All these countries border the Pacific Ocean, which is the most hydrate-rich zone [
6,
7]. Gas hydrate deposits were also discovered in South China Sea and on the Arctic seafloor. In the World Ocean, gas hydrates are most often discovered on continental slopes and shelves [
8]. This is attributed to the accumulation of sediments with elevated content of organic matter, which is a source of thermogenic and microbial methane [
9]. If there is a source of methane, marine gas hydrates are formed at a depth of over 320 m. The depth of the seafloor at which hydrates are formed depends on the bottom water temperature: the higher the temperature, the greater the sea depth is needed for hydrate formation [
10]. The sea level reduction and increase in the bottom water temperature leads to the destruction of gas hydrates and release of methane into water and the atmosphere [
11] with an adverse effect on the environment. Researching gas hydrates is thus a topical objective in terms of environmental protection. A comprehensive review on studies of natural gas hydrates is proposed in [
4].
Discovery of gas hydrate deposits is a crucial and complicated part of gas hydrate research. Gas hydrates can be detected by hydroacoustic survey, seismic sounding, geophysical measurements, and electromagnetic exploration [
12,
13,
14]. The most popular method of hydrate detection on the seabed and ocean floor is standard and high-frequency seismic exploration [
13,
15]. Seismic surveys can be two-dimensional (2D) and three-dimensional (3D) [
16]. The 2D survey data reveal the lower position of hydrate-bearing sediments with a frequency of 30–120 Hz. A 3D seismic survey determines the top and bottom boundaries of hydrate-bearing sediments and provides data on the hydrate concentration in the sediments. Geophysical measurements are extra sensitive to gas hydrates but technically challenging [
17]. When marine controlled-source electromagnetics is used, the data on hydrate-saturated sediments are acquired using transmitters on the seafloor and the corresponding receiver [
12]. Drilling for gas hydrates using specialized rigs (Japan, Korea, and China) has also become popular over the recent years [
18,
19].
In addition to gas hydrate detection methods, researchers focus on the technologies of natural gas production from hydrates, hydrate dissociation, and self-preservation, as well as hydrate ignition and combustion behavior [
20]. Natural gas can be recovered from hydrates by means of three main technologies [
4,
21,
22,
23,
24,
25]: using inhibitors, thermal method, and combined method. Injecting inhibitors into a hydrate reservoir disturbs the phase equilibrium of gas hydrates [
26]. Saline or organic solutions can serve as inhibitors. The thermal method makes it possible to produce gaseous methane at a rate that is an order of magnitude higher than what depressurization and inhibitor injection can provide [
27]. However, thermal stimulation requires a detailed study of the dissociation process and gas flow dynamics. The combined method involving depressurization and thermal exposure at the same time is considered the most promising one [
24]. Heat can be supplied in the form of electric heating or hot water or vapor. Hot water injection was found [
24] to enhance thermal convection and to accelerate the gas hydrate dissociation.
The understanding of the dissociation process is an important part of gas hydrate research. The dissociation kinetics affects both the natural gas production from hydrates, and the ignition and combustion behavior. In turn, the gas hydrate dissociation rate is affected by a multitude of factors: hydrate particle size, type of hydrate, pressure, and temperature. Research findings on the dissociation kinetics of natural gas hydrates from marine hydrate deposits are presented in [
28]. Higher temperature and mixing speed significantly stimulated the dissociation of gas hydrate, whereas the granule size exhibited a negligible effect. In addition, a highly accurate kinetic model was constructed to describe the methane hydrate dissociation, which simultaneously reflected the impact of pressure, temperature, and mixing speed on the seemingly constant hydrate dissociation rate [
28]. The authors in [
29,
30] showed that the methane hydrate dissociation rate varies by up to 5 orders of magnitude in the temperature range of 230–268 K. The impact of temperature and external pressure on the dissociation rate was studied by Stern et al. [
31]. Misyura [
32] investigated the dissociation of different gas hydrates during their combustion. It was established [
32] that the dissociation rate of methane–propane double hydrate is higher than that of methane hydrate. The dissociation rate of methane–isopropanol hydrate is much lower than that of methane hydrate and methane–propane double hydrate. The flame front velocity is highly nonlinear due to the gas hydrate self-preservation effect, which emerges in the course of dissociation at negative temperatures [
33]. This is a reduction in the rate of hydrate decomposition into gas and water up to its complete stop [
34]. According to Stern et al. [
31], the gas hydrate self-preservation is based on the morphological changes inside the hydrate.
Not only dissociation but also combustion of gas hydrate is a subject of large-scale research [
35,
36,
37,
38]. The combustion of methane hydrate has been poorly studied due to the complex characteristics of the multiphase transition and the difficulty of working with fuel that decomposes at standard temperature and pressure [
39]. The combustion process can be subdivided into the following stages: ice shell melting, water evaporation, and flammable component combustion [
35]. The main feature of gas hydrate combustion is the hydrate-dissociated reaction of the gas because such fuels consist of gas and water molecules. The water content of the fuel has a significant effect on its stability, ignition, and combustion characteristics. The effect of the introduction of hydrous ethanol with different water content on the operation of a diesel engine was studied in [
40]. The effect of water on the stability of isopropanol–butanol–ethanol (IBE) and diesel fuel was studied in [
41]. It has been established that the use of a small amount of water can, to a certain extent, improve the environmental and energy performance of a diesel engine. Water evaporation and water vapor formation are considered the main factors influencing the hydrate combustion kinetics. A water film is formed at the surface of gas hydrate while it is burning [
42]. A part of water evaporates and reaches the flame zone. Water also partially drains down through the porous space between hydrate particles. The thickness of the water film governs the heat exchange between the combustion source and the gas hydrate surface. It depends on the ice melting rate, water evaporation rate, convection over the film, as well as drainage rate [
42]. With an increase in the water vapor concentration from 0% to 70%, the total methane flow decreased by almost one order of magnitude. Lower vapor content in the gas mixture leads to a considerable increase in the dissociation rate [
42]. The propane hydrate combustion time increases at high hydrate saturation and porosity [
36], and water formed from the hydrate dissociation plays a key part in this process: it restrains the heat transfer from the flame to the hydrate and prevents the gas from releasing to the combustion zone [
36]. The impact of water on the flame characteristics of methane hydrate combustion was studied in [
38]. Water vapor released during the hydrate combustion decreases in the flame temperature compared to the combustion of pure methane, and its mechanistic action changes the water response to some elementary reactions. The research findings [
38] revealed that the addition of water vapor significantly changed the reaction behavior of some elementary reactions (chemical effect), which led to a reduction in flame temperature. However, the concentration of the intermediate element components decreased as well (physical effect), thus inhibiting the combustion rate [
38]. At present, the direct combustion of gas hydrate has been actively studied. Dunn-Rankin [
43] proposed the concept of direct energy conversion by on-site combustion of methane hydrates and carbon dioxide sequestration in the deep ocean. A new cylindrical porous burner for burning gas hydrate was proposed in [
39] to solve the problem of the appearance of a water film and the self-preservation effect. The proposed burner makes it possible to efficiently use methane hydrate and maintain a stable flame during the combustion process. Using such a counterflow burner, the combustion of methane hydrate can theoretically be categorized as a diffuse methane flame with a large amount of water vapor in the fuel stream. Wu and Chao [
44] also proposed a burner for maintaining stable combustion of methane hydrate. It was established that the flame during the combustion of methane hydrate using the proposed burner has an ideal round shape and is similar to a simple symmetrical diffusion flame [
44]. The measured gas release rate was about 7.5 mg/s and the average flame temperature of methane hydrate was about 1650 K [
44]. The mass fraction of water vapor in the flame of methane hydrate was no more than 80% [
44]. Chien and Dunn-Rankin [
45] ignited a methane hydrate sample using a piezo igniter and a butane lighter. An ignition device with an open crucible was also used. As a result of the study, the geometry of the flame was obtained from the moment of ignition of the methane hydrate to the moment of its extinguishing. Direct combustion of propane hydrate in a combustion chamber with an electronic igniter was considered in [
36]. Chen et al. [
36] found that high rates of hydrate dissociation and gas release favor the combustion process. Hydrate-dissociated water plays an important role in this process since the accumulation of water significantly limits both the transfer of heat from the flame to the hydrates and the release of gas into the combustion zone.
Among the multitude of research fields associated with gas hydrates, the study of their ignition and combustion as high-potential fuels is of special interest. It is advisable to study the impact of water in hydrate on the key combustion stages and the environmental friendliness of the whole process.
1.2. Coal–Water Slurries
Production of coal–water slurries (CWS) is an alternative way to involve water-based resources in the power-generating sector. These fuels are a mixture of coal components and water with mass concentrations ranging from 35 to 65 wt% [
46,
47]. Special-purpose additives (biomass [
48], used oils [
49], oil sludge [
50], etc.) in the amount of 5–20 wt% can also be included to increase or reduce certain characteristics, for instance, energy or environmental ones. Such fuels can be produced from a wide range of resources. In particular, coals of different quality (high-rank anthracite or low-rank brown coals) [
51,
52] and their processing waste (coal slime, middlings, etc.) can be used as the solid component [
47,
53]. Tap water can serve as the liquid inert constituent. However, a number of research teams used service water [
54], liquid industrial waste [
55,
56], and sewage sludge [
57,
58] to produce CWS. For instance, the authors in [
54] presented experimental research into the impact of water quality on coal–water slurry ignition behavior. The authors used service water obtained from the city power station, tap water, and distilled water. It was experimentally established that CWS based on service water exhibited 8% faster ignition and higher combustion temperatures as compared to fuels based on purified water. This was attributed to the presence of flammable additives and effluents in service water. Their evaporation and combustion accelerated the heating and ignition of coke residue. However, the differences in the ignition delay times between composite fuels based on water of different quality are rather limited: they do not exceed 3% at oxidizer temperatures above 600 °C [
54]. Lei et al. [
58] studied the co-combustion of sewage sludge and bituminous coal in air. An increase in the proportion of sewage sludge from 0% to 50% shortened the ignition delay time and burnout time of volatiles [
58]. Liu et al. [
56] presented the experimental research into the combustion of coal–water slurries containing liquid petrochemical waste instead of water. The experimental findings showed that slurries containing liquid petrochemical waste exhibited lower viscosity, faster ignition, higher flame temperature, and lower NO
x and SO
x emissions compared to conventional coal–water slurries [
56]. Thus, depending on the conditions and requirements, service water can be used for CWS production without treatment. This approach is rational as it saves pure water and makes use of service water, which would otherwise be dumped.