Measurement of Instantaneous Mass Flow Rate of Polypropylene Gasification Products in Airflow
Abstract
:1. Introduction
2. Experimental Setup
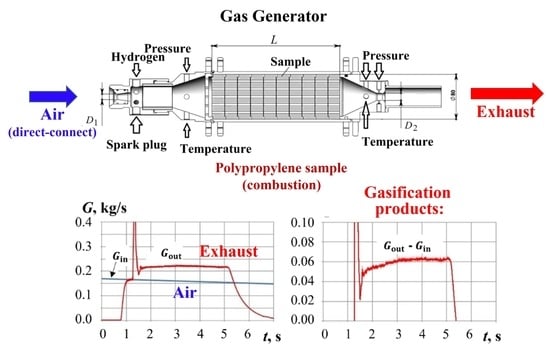
2.1. Gas Generator with Hydrogen-Assisted Ignition
2.2. Gas Generator with Pyro-Charge-Assisted Ignition
2.3. Measurements
3. Data Processing Procedure
3.1. Mass Flow Rate at GG Inlet
3.2. Mass Flow Rate at GG Outlet
3.3. Mass Flow Rate of Gasification Products
4. Typical Test Fires
4.1. Test Fires with Hydrogen-Assisted Ignition
4.2. Test Fires with Pyro-Charge-Assisted Ignition
5. Results and Discussion
5.1. Yield of Gasification Products with Hydrogen-Assisted Ignition
5.2. Yield of Gasification Products with Pyro-Charge-Assisted Ignition
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sutton, G.P.; Biblarz, O. Rocket Propulsion Elements, 9th ed.; Wiley: New York, NY, USA, 2017. [Google Scholar]
- Freesmeier, J.J.; Butler, P.B. Analysis of a Hybrid Dual-Combustion-Chamber Solid-Propellant Gas Generator. J. Propuls. Power 1999, 15, 552–561. [Google Scholar] [CrossRef]
- Karabeyoglu, A.; Zilliac, G.; Cantwell, B.J.; DeZilwa, S.; Castellucci, P. Scale-up Tests of High Regression Rate Paraffin-Based Hybrid Rocket Fuels. J. Propuls. Power 2004, 20, 1037–1045. [Google Scholar] [CrossRef]
- Galfetti, L.; Merotto, L.; Boiocchi, M.; Maggi, F.; DeLuca, L.T. Experimental Investigation of Paraffin-Based Fuels for Hybrid Rocket Propulsion. In Progress in Propulsion Physics; DeLuca, L., Bonnal, C., Haidn, O., Frolov, S., Eds.; EUCASS Advances in Aerospace Sciences Book Series; EDP Sciences–Torus Press: Les Ulis, France, 2013; Volume 4, pp. 59–74. [Google Scholar] [CrossRef] [Green Version]
- Mazzetti, A.; Merotto, L.; Pinarello, G. Paraffin-Based Hybrid Rocket Engines Applications: A Review and a Market Perspective. Acta Astronaut. 2016, 126, 286–297. [Google Scholar] [CrossRef]
- Wada, Y.; Kawabata, Y.; Kato, R.; Kato, N.; Hori, K. Observation of Combustion Behavior of Low Melting Temperature Fuel for a Hybrid Rocket Using Double Slab Motor. Int. J. Energetic Mater. Chem. Propuls. 2016, 15, 351–369. [Google Scholar] [CrossRef]
- Lee, D.; Lee, C. Hybrid Gas Generator for a Staged Hybrid Rocket Engine. J. Propuls. Power 2017, 33, 204–212. [Google Scholar] [CrossRef]
- Wang, L.; Wu, Z.; Chi, H.; Liu, C.; Tao, H.; Wang, Q. Numerical and Experimental Study on the Solid-Fuel Scramjet Combustor. J. Propuls. Power 2015, 31, 685–693. [Google Scholar] [CrossRef]
- Zvegintsev, V.I.; Fedorychev, A.V.; Zhesterev, D.V.; Mishkin, I.R.; Frolov, S.M. Gasification of Low-Melting Hydrocarbon Materials in High-Temperature Gas Flow. Combust. Explos. 2019, 12, 108–116. [Google Scholar] [CrossRef]
- Frolov, S.M.; Shamshin, I.O.; Kazachenko, M.V.; Aksenov, V.S.; Bilera, I.V.; Ivanov, V.S.; Zvegintsev, V.I. Polyethylene Pyrolysis Products: Their Detonability in Air and Applicability to Solid-Fuel Detonation Ramjets. Energies 2021, 14, 820. [Google Scholar] [CrossRef]
- Schmitt, R.G.; Butler, P.B.; Freesmeier, J.J. Performance and CO Production of a Non-Azide Airbag Propellant in a Pre-Pressurized Gas Generator. Combust. Sci. Technol. 1997, 122, 305–330. [Google Scholar] [CrossRef]
- Wu, W.T.; Hsieh, W.H.; Huang, C.H.; Wang, C.H. Theoretical Simulation of Combustion and Inflation Processes of Two-Stage Airbag Inflators. Combust. Sci. Technol. 2005, 177, 383–412. [Google Scholar] [CrossRef]
- Cheng, C.; Zhang, X.; Wang, C.; Wang, L. Numerical Investigation on Cooling Performance of Filter in a Pyrotechnic Gas Generator. Def. Technol. 2021, 17, 343–351. [Google Scholar] [CrossRef]
- Schmid, H.; Eisenreich, N.; Baier, A.; Neutz, J.; Schröter, D.; Weiser, V. Gas Generator Development for Fire Protection Purpose. Propellants Explos. Pyrotech. 1999, 24, 144–148. [Google Scholar] [CrossRef]
- Mahinpey, N.; Gomez, A. Review of Gasification Fundamentals and New Findings: Reactors, Feedstock, and Kinetic Studies. Chem. Eng. Sci. 2016, 148, 14–31. [Google Scholar] [CrossRef]
- Salaudeen, S.A.; Arku, P.; Dutta, A. Gasification of Plastic Solid Waste and Competitive Technologies. In Plastics to Energy; Al-Salem, S.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 269–293. [Google Scholar] [CrossRef]
- Salgansky, E.A.; Lutsenko, N.A. Effect of Solid Fuel Characteristics on Operating Conditions of Low-Temperature Gas Generator for High-Speed Flying Vehicle. Aerosp. Sci. Technol. 2021, 109, 106420. [Google Scholar] [CrossRef]
- Rashkovskiy, S.A.; Yakush, S.E. Numerical Simulation of Low-Melting Temperature Solid Fuel Regression in Hybrid Rocket Engines. Acta Astronaut. 2020, 176, 710–716. [Google Scholar] [CrossRef]
- DeLuca, L.T.; Galfetti, L.; Colombo, G.; Maggi, F.; Bandera, A.; Boiocchi, M.; Gariani, G.; Merotto, L.; Paravan, C.; Reina, A. Time-Resolved Burning of Solid Fuels for Hybrid Rocket Propulsion. In Progress in Propulsion Physics; DeLuca, L., Bonnal, C., Haidn, O., Frolov, S., Eds.; EUCASS Advances in Aerospace Sciences Book Series; EDP Sciences–Torus Press: Les Ulis, France, 2011; Volume 2, pp. 405–426. [Google Scholar] [CrossRef] [Green Version]
- Shiplyuk, A.N.; Zvegintsev, V.I.; Frolov, S.M.; Vnuchkov, D.A.; Kiseleva, T.A.; Kislovsky, V.A.; Lukashevich, S.V.; Melnikov, A.Y.; Nalivaychenko, D.G. Gasification of Low-Melting Hydrocarbon Material in the Airflow Heated by Hydrogen Combustion. Int. J. Hydrogen Energy 2020, 45, 9098–9112. [Google Scholar] [CrossRef]
- Shiplyuk, A.N.; Zvegintsev, V.I.; Frolov, S.M.; Vnuchkov, D.A.; Kislovsky, V.A.; Kiseleva, N.A.; Lukashevich, S.V.; Melnikov, A.Y.; Nalivaychenko, D.G. Gasification of Low-Melting Fuel in a High-Temperature Flow of Inert Gas. J. Propuls. Power 2021, 37, 20–28. [Google Scholar] [CrossRef]
- Arkhipov, V.A.; Basalaev, S.A.; Kuznetsov, V.T.; Poryazov, V.A.; Fedorychev, A.V. Modeling of Ignition and Combustion of Boron-Containing Solid Propellants. Combust. Explos. Shock. Waves 2021, 57, 308–313. [Google Scholar] [CrossRef]
- Zarko, V.; Perov, V.; Kiskin, A.; Nalivaichenko, D. Microwave Resonator Method for Measuring Transient Mass Gasification Rate of Condensed Systems. Acta Astronaut. 2019, 158, 272–276. [Google Scholar] [CrossRef]
- Evans, B.N.; Favorito, A.; Kuo, K. Study of Solid Fuel Burning-Rate Enhancement Behavior in an X-ray Translucent Hybrid Rocket Motor. In AIAA Paper 2005-3909, Proceedings of the 41st AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, Tucson, Arizona, USA, 10–13 July 2005; The American Institute of Aeronautics and Astronautics: Reston, VA, USA, 2012. [Google Scholar] [CrossRef]
- Saito, Y.; Kamps, L.T.; Komizu, K.; Bianchi, D.; Nasuti, F.; Nagata, H. The accuracy of reconstruction techniques for determining hybrid rocket fuel regression rate. In AIAA Paper 2018-4923, AIAA Propulsion and Energy Forum, Proceedings of the Joint Propulsion Conference, Cincinnati, OH, USA, 9–11 July 2018; The American Institute of Aeronautics and Astronautics: Reston, VA, USA, 2018. [Google Scholar] [CrossRef]
- Zvegintsev, V.I. Short-Duration Gas-Dynamic Facilities. Part 1. Facilities for Scientific Research; Parallel Publ.: Novosibirsk, Russia, 2014; (In Russian). ISBN 978-5-98901-169-8. [Google Scholar]
- Trusov, B.G. Modeling of Chemical and Phase Equilibria at High Temperatures “Astra 4”; Bauman State Technical University Publ.: Moscow, Russia, 1991. [Google Scholar]














| Test Fire | 2 | 3 |
|---|---|---|
| Start of ignition, s | 1.53 | 1.53 |
| End of ignition, s | 2.05 | 2.05 |
| Air mass flow rate, g/s | 232.7 | 232.1 |
| Mass of air, g | 121.0 | 120.7 |
| Mass flow rate of gasification products, g/s | 120.8 | 106.9 |
| Yield of gasification products, g | 62.8 | 55.6 |
| Ratio of mass flow rates of air and gasification products | 1.93 | 2.17 |
| Start of combustion, s | 2.05 | 2.05 |
| End of combustion, s | 5.40 | 5.20 |
| Air mass flow rate, g/s | 211.3 | 219.9 |
| Mass of air, g | 708.0 | 692.8 |
| Mass flow rate of gasification products, g/s | 82.1 | 72.2 |
| Yield of gasification products, g | 274.9 | 227.4 |
| Ratio of mass flow rates of air and gasification products | 2.58 | 3.05 |
| Total during test fire: | ||
| Air mass flow rate, g/s | 214.2 | 221.7 |
| Mass of air, g | 829.0 | 813.5 |
| Mass flow rate of gasification products, g/s | 87.2 | 77.1 |
| Yield of gasification products, g | 337.6 | 283.0 |
| Ratio of mass flow rates of air and gasification products | 2.46 | 2.87 |
| Test Fire | 6 | 7 | 8 | 9 |
|---|---|---|---|---|
| Start of combustion, s | 1.30 | 1.25 | 1.33 | 1.32 |
| End of combustion, s | 5.10 | 5.37 | 5.40 | 5.48 |
| Air mass flow rate, g/s | 166.4 | 159.79 | 156.4 | 124.8 |
| Mass of air, g | 632.4 | 658.0 | 636.4 | 519.3 |
| Mass flow rate of gasification products, g/s | 71.2 | 67.0 | 65.0 | 43.0 |
| Yield of gasification products, g | 270.7 | 276.2 | 264.7 | 179.0 |
| Ratio of mass flow rates of air and gasification products | 2.34 | 2.38 | 2.40 | 2.90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vnuchkov, D.A.; Zvegintsev, V.I.; Nalivaichenko, D.G.; Frolov, S.M. Measurement of Instantaneous Mass Flow Rate of Polypropylene Gasification Products in Airflow. Energies 2022, 15, 5765. https://doi.org/10.3390/en15165765
Vnuchkov DA, Zvegintsev VI, Nalivaichenko DG, Frolov SM. Measurement of Instantaneous Mass Flow Rate of Polypropylene Gasification Products in Airflow. Energies. 2022; 15(16):5765. https://doi.org/10.3390/en15165765
Chicago/Turabian StyleVnuchkov, Dmitry A., Valery I. Zvegintsev, Denis G. Nalivaichenko, and Sergey M. Frolov. 2022. "Measurement of Instantaneous Mass Flow Rate of Polypropylene Gasification Products in Airflow" Energies 15, no. 16: 5765. https://doi.org/10.3390/en15165765
APA StyleVnuchkov, D. A., Zvegintsev, V. I., Nalivaichenko, D. G., & Frolov, S. M. (2022). Measurement of Instantaneous Mass Flow Rate of Polypropylene Gasification Products in Airflow. Energies, 15(16), 5765. https://doi.org/10.3390/en15165765