Composition of Flue Gases during Oxy-Combustion of Energy Crops in a Circulating Fluidized Bed
Abstract
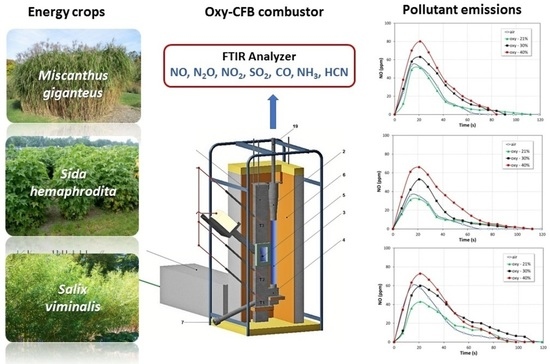
:1. Introduction
2. Materials and Methods
2.1. Fuel Tested
2.2. Experimental Apparatus and Procedure
3. Results and Discussion
3.1. Composition of Flue Gases during Air-Combustion
3.2. Composition of Flue Gases during Oxy-Combustion
4. Conclusions
- Sida hermaphrodita is the most environmentally friendly energy crop for both air and oxy-CFB combustion.
- The instantaneous emissions of NO, N2O, CO, and SO2 for the combustion of energy crops in O2/CO2 environments are lower than those for combustion of agricultural biomass (wheat straw) and higher than those for combustion of woody biomass (Scots pine).
- The instantaneous NO emissions during the combustion of energy crops in all atmospheres are higher than those of hard coal, which is due to the much higher content of volatile matter in renewable fuels.
- The oxidation of volatile nitrogen compounds is behind high emissions of NOx from energy crops burned in air and O2/CO2 mixtures.
- The highest values of the instantaneous concentrations of NO and N2O for energy crops during oxy-combustion are observed for Miscanthus giganteus and during air-combustion for Salix viminalis.
- Combustion of energy crops in the oxy-21% atmosphere causes the lowest NO emissions and the highest N2O emissions, which is associated with a lower temperature of burning fuel samples.
- Emissions of CO for the combustion of energy crops in all atmospheres are much lower than those for the combustion of reference coal. It can be attributed to the higher content of carbon in fossil fuel.
- Combustion of energy crops in the mixture of 21%O2 and 79%CO2 results in a very large increase in CO concentrations in the exhaust gas compared to conventional combustion.
- As the initial concentration of oxygen in the O2/CO2 mixture increases, emissions of SO2 and NO increase even though emissions of CO and N2O decrease for energy crops and reference fuels.
- Considering all emitted pollutants, the optimal atmosphere for the oxy-CFB combustion of energy crops should contain oxygen in the range of 21–30%vol.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pradhan, P.; Mahajani, S.M.; Arora, A. Production and utilization of fuel pellets from biomass: A review. Fuel Process. Technol. 2018, 181, 215–232. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An overview of the chemical composition of biomass. Fuel 2010, 89, 913–933. [Google Scholar] [CrossRef]
- Halford, N.G.; Karp, A. (Eds.) Energy Crops. RSC Energy and Environment Series No. 3; Royal Society of Chemistry: London, UK, 2011. [Google Scholar]
- Anthony, E.J. Fluidized bed combustion of alternative solid fuels; status, successes and problems of the technology. Prog. Energy Combust. Sci. 1995, 21, 236–268. [Google Scholar] [CrossRef]
- Giglio, R.; Castilla, N.J. The value proposition of circulating fluidized-bed technology for the utility power sector. J. Southern African Inst. Mining Metall. 2015, 115, 581–588. [Google Scholar] [CrossRef]
- Basu, P. Circulating Fluidized Bed Boilers. Design, Operation and Maintenance; Springer: Cham, Switzerland; Heidelberg, Germany, 2015. [Google Scholar]
- Natunen, M.; Jäntti, T.; Goral, D.; Nuortimo, K. First operating experiences of 55 MWe Konin and 205 MWe Połaniec CFB boilers firing 100% biomass. In Proceedings of the Paper presented at PowerGen Europe conference, Vienna, Austria, 4–6 June 2013. [Google Scholar]
- Giglio, R.; Jäntti, T. Tees Renewable Energy Plant. Mod. Power Syst. 2017, 21–26. [Google Scholar]
- Jia, L.; Tan, Y.; Anthony, E.J. Emissions of SO2 and NOx during oxy-fuel CFB combustion in a mini-circulating fluidized bed combustion reactor. Energy Fuels 2010, 24, 910–915. [Google Scholar] [CrossRef]
- Li, L.; Duan, L.; Yang, Z.; Zhao, C. Pressurized oxy-fuel combustion characteristics of single coal particle in a visualized fluidized bed combustor. Comb. Flame 2020, 211, 218–228. [Google Scholar] [CrossRef]
- Liang, X.; Wang, Q.; Luo, Z.; Eddings, E.; Ring, T.; Li, S.; Yu, P.; Yan, J.; Yang, X.; Jia, X. Experimental and numerical investigation on nitrogen transformation in pressurized oxy-fuel combustion of pulverized coal. J. Clean. Prod. 2021, 278, 123240. [Google Scholar] [CrossRef]
- Kosowska-Golachowska, M.; Luckos, A.; Kijo-Kleczkowska, A. Pollutant emissions during oxy-fuel combustion of biomass in a bench scale CFB combustor. Energies 2022, 15, 706. [Google Scholar] [CrossRef]
- Tan, T.; Jia, L.; Wu, Y. Some combustion characteristics of biomass and coal cofiring under oxy-fuel conditions in a pilot-scale circulating fluidized combustor. Energy Fuels 2013, 27, 7000–7007. [Google Scholar] [CrossRef]
- Duan, L.; Duan, Y.; Zhao, C.; Anthony, E.J. NO emission during co-firing coal and biomass in an oxy-fuel circulating fluidized bed combustor. Fuel 2015, 150, 8–13. [Google Scholar] [CrossRef]
- Wang, X.; Ren, Q.; Li, W.; Li, H.; Li, S.; Lu, Q. Nitrogenous gas emissions from coal/biomass co-combustion under a high oxygen concentration in a circulating fluidized bed. Energy Fuels 2017, 31, 3234–3242. [Google Scholar] [CrossRef]
- Varol, M.; Symonds, R.; Anthony, E.J.; Lu, D.; Jia, L.; Tan, Y. Emissions from co-firing lignite and biomass in an oxy-fired CFBC. Fuel Process. Technol. 2018, 173, 126–133. [Google Scholar] [CrossRef]
- Sun, J.-H.; Back, S.-K.; Jeong, B.-M.; Kim, J.-H.; Choi, H.S.; Jang, H.-N.; Seo, J.-C. Oxy-fuel co-combustion of sewage sludge and wood pellets with flue gas recirculation in a circulating fluidized bed. Fuel Process. Technol. 2018, 172, 79–85. [Google Scholar]
- Nguyen, H.K.; Moon, J.-H.; Jo, S.-H.; Park, S.J.; Seo, M.W.; Ra, H.W.; Yoon, S.-J.; Yoon, S.-M.; Song, B.; Lee, U.; et al. Oxy-combustion characteristics as a function of oxygen concentration and biomass co-firing ratio in a 0.1 MWth circulating fluidized bed combustion test-rig. Energy 2020, 196, 117020. [Google Scholar] [CrossRef]
- Moreno, J.; Hornberger, M.; Schmid, M.; Scheffknecht, G. Oxy-fuel combustion of hard coal, wheat straw, and solid recovered fuel in a 200 kWth calcium looping CFB calciner. Energies 2021, 14, 2162. [Google Scholar] [CrossRef]
- Meng, X.; Rokni, E.; Zhou, W.; Qi, H.; Sun, R.; Levendis, Y.A. Emissions from oxy-combustion of raw and torrefied biomass. J. Energy Resour. Technol. 2020, 142, 122307. [Google Scholar] [CrossRef]
- Kosowska-Golachowska, M.; Kijo-Kleczkowska, A.; Luckos, A.; Wolski, K.; Musiał, T. Oxy-combustion of biomass in a circulating fluidized bed. Arch. Thermodyn. 2016, 37, 17–30. [Google Scholar] [CrossRef]
- Kosowska-Golachowska, M.; Luckos, A.; Musiał, T.; Kijo-Kleczkowska, A.; Wolski, K.; Środa, K. NOx and N2O emissions during oxy-fuel combustion of bituminous coal and lignite in a circulating fluidized bed combustor. In Proceedings of the 13th International Conference on Fluidized Bed Technology, Vancouver, BC, Canada, 10–14 May 2021; pp. 587–592. [Google Scholar]
- Kosowska-Golachowska, M.; Luckos, A.; Kijo-Kleczkowska, A.; Musiał, T.; Wolski, K.; Środa, K. Gaseous emissions during oxy-fuel combustion of sewage sludge in a circulating fluidized bed. Powder Technol. 2020, 371, 209–216. [Google Scholar] [CrossRef]
- Kijo-Kleczkowska, A.; Środa, K.; Kosowska-Golachowska, M.; Musiał, T.; Wolski, K. Combustion of pelleted sewage sludge with reference to coal and biomass. Fuel 2016, 170, 141–160. [Google Scholar] [CrossRef]
- Wolski, K. Experimental investigation into oxy-fuel combustion of agro and wooden biomass in a circulating fluidized bed. Ph.D. Thesis, Czestochowa University of Technology, Czestochowa, Poland, 2016. (In Polish). [Google Scholar]
- Czakiert, T.; Sztekler, K.; Karski, S.; Markiewicz, D.; Nowak, W. Oxy-fuel circulating fluidized bed combustion in a small pilot-scale test rig. Fuel Processing Technol. 2010, 91, 1617–1623. [Google Scholar] [CrossRef]
- Jankowska, S.; Czakiert, T.; Krawczyk, G.; Borecki, P.; Jesionowski, Ł.; Nowak, W. The effect of oxygen staging on nitrogen conversion in oxy-fuel CFB environment. Chem. Process Eng. 2014, 35, 489–496. [Google Scholar] [CrossRef]
- Lasek, J.A.; Janusz, M.; Zuwała, J.; Głód, K.; Iluk, A. Oxy-fuel combustion of selected solid fuels under atmospheric and elevated pressures. Energy 2013, 62, 105–112. [Google Scholar] [CrossRef]






| Reference | Test Facility and Operating Conditions | Fuels | Oxidizing Medium | Emissions Reported | Remarks |
|---|---|---|---|---|---|
| Tan et al. [13] | CFB, 800 kWth, ~900 °C, limestone, Ca/S = 3 | Wood pellets, different coals, fraction of biomass in fuel blends 20–50%wt. | O2/CO2 mixtures, 24–25% O2, recycled flue gas | NOx, SO2, CO, CO2, O2 | The addition of wood pellets did not have a notable impact on the combustion characteristics of tested fuels. Emissions of NO (per unit of energy (higher heating value) in the fuel) were in the range of 14–20 ng/J, and emissions of SO2 varied from 35 to 95 ng/J. When the O2 concentration in the flue gas exceeded 3.5%, emissions of CO dropped below 200 ppm. |
| Duan et al. [14] | CFB, 10 kWth, 850 ± 10 °C | Rice husk, wood chips, dry wood flour, coal, biomass fraction in fuel 0–100%wt. | Air, 70% O2/30% CO2 mixture | NO, NO2, CO, CO2, O2, SO2 | Emissions of NO were higher for biomass fuels than for coal. Oxy-combustion produced less NO than combustion in air. NO emissions in the air and oxy-fuel atmosphere increased with an increasing fraction of biomass in the fuel blend. |
| Wang et al. [15] | CFB, 10 kWth, 800–900 °C | Corn straw, wheat straw, coal, 30% biomass in fuel blend | 50% O2/50% CO2, 50% O2/50% recycled flue gas | NO, N2O, CO, CO2, O2, HCN | NO and N2O emissions increased with an increasing excess O2. An increase in the fraction of corn straw in the fuel blend caused an increase in emission factors of NO, N2O, and HCN. |
| Varol et al. [16] | CFB, 850 and 915 °C, limestone, Ca/S = 2 | Wood pellets, high-sulphur lignite, biomass/lignite ratio up to 60% | 25, 30% O2, CO2 | NOx, SO2, CO, CO2 | Increasing biomass share in the fuel blend had a negligible effect on NOx emissions. Emissions of CO and SO2 decreased with an increasing fraction of biomass in the fuel blend. |
| Sung et al. [17] | CFB, 30 kWth, 750–840 °C | Sewage sludge, wood pellets | O2 with recirculated flue gas | CO, NO | The lowest concentrations of CO (0.91%) and NO (14 ppm) were at 60% flue gas recirculation. |
| Nguyen et al. [18] | CFB, 100 kWth, 845–905 °C, flue gas recirculation | Wood pellets, lignite, biomass fraction in fuel blend 50–100%wt. | 21–29% O2, CO2 | NO, SO2, CO | An increase in biomass share caused a decrease in NO, SO2 and CO concentrations. Oxy-combustion of pure biomass can produce negative CO2 emissions of, approximately, –647 g/kWth. |
| Moreno et al. [19] | CFB, 200 kWth, 910 ± 10 °C, 835–852 °C, limestone | Coal, wheat straw, solid recovered fuel | O2, recirculated flue gas | NOx, SO2, HCl | NOx emissions of 359 mg/MJth for combustion of coal and 203 mg/MJth for co-combustion of coal with biomass. Emissions of SO2 were 1.9–2.0 mg/MJth. Emissions of HCl were 1.8 mg/MJth for tests with no wheat straw and 3.9 mg/MJth for combustion of pure biomass. |
| Miscanthus giganteus | Sida hermaphrodita | Salix viminalis | Wheat Straw [12] | Scots Pine [12] | Bituminous Coal [12] | |
|---|---|---|---|---|---|---|
| Proximate analysis (wt.%, db) | ||||||
| Moisture | 6.0 | 6.3 | 6.9 | 8.4 | 7.0 | 8.7 |
| Ash | 12.4 | 9.4 | 1.4 | 6.1 | 0.6 | 18.9 |
| Volatile matter | 75.8 | 76.7 | 76.3 | 68.3 | 76.8 | 26.8 |
| Fixed carbon * | 5.8 | 7.6 | 15.4 | 17.2 | 15.6 | 45.6 |
| Higher heating value, MJ/kg | 17.73 | 17.53 | 18.20 | 17.84 | 18.90 | 22.75 |
| Ultimate analysis (wt.%, daf) | ||||||
| Carbon | 46.02 | 44.78 | 49.60 | 50.20 | 50.90 | 73.30 |
| Hydrogen | 5.38 | 5.19 | 6.00 | 5.80 | 5.70 | 4.30 |
| Sulphur | 0.03 | 0.02 | 0.03 | 0.08 | 0.01 | 2.30 |
| Nitrogen | 0.15 | 0.08 | 0.30 | 0.80 | 0.10 | 1.10 |
| Chlorine | 0.08 | 0.01 | 0.01 | 0.15 | 0.01 | 0.70 |
| Oxygen * | 48.34 | 49.92 | 44.06 | 42.97 | 43.28 | 18.30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosowska-Golachowska, M.; Luckos, A.; Czakiert, T. Composition of Flue Gases during Oxy-Combustion of Energy Crops in a Circulating Fluidized Bed. Energies 2022, 15, 6889. https://doi.org/10.3390/en15196889
Kosowska-Golachowska M, Luckos A, Czakiert T. Composition of Flue Gases during Oxy-Combustion of Energy Crops in a Circulating Fluidized Bed. Energies. 2022; 15(19):6889. https://doi.org/10.3390/en15196889
Chicago/Turabian StyleKosowska-Golachowska, Monika, Adam Luckos, and Tomasz Czakiert. 2022. "Composition of Flue Gases during Oxy-Combustion of Energy Crops in a Circulating Fluidized Bed" Energies 15, no. 19: 6889. https://doi.org/10.3390/en15196889
APA StyleKosowska-Golachowska, M., Luckos, A., & Czakiert, T. (2022). Composition of Flue Gases during Oxy-Combustion of Energy Crops in a Circulating Fluidized Bed. Energies, 15(19), 6889. https://doi.org/10.3390/en15196889