Prediction of the Total Base Number (TBN) of Engine Oil by Means of FTIR Spectroscopy
Abstract
:1. Introduction and Theoretical Background
2. Materials and Methods
2.1. Research Material
2.2. Research Methodology
3. Results
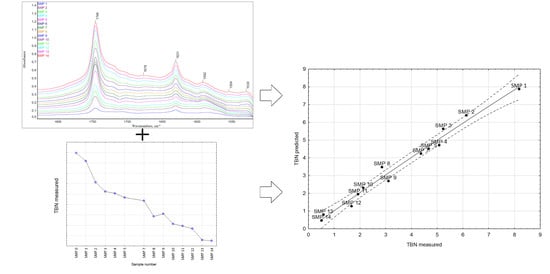
3.1. The FTIR Results
3.2. The TBN Results
4. Modeling TBN Levels Based on the FTIR Spectra
- Model A—the model was built on the basis of average absorbance values calculated in four wavenumber ranges of the same width, i.e., <1000 cm−1, 1000–2000 cm−1, 2000–3000 cm−1, and >3000 cm−1.
- Model B—the model was elaborated on the basis of average absorbance values in two key wavenumber ranges, i.e., 1000–1450 cm−1 and 1470–1800 cm−1, in which the greatest differences in the intensity of spectral bands could be identified, related to the changes in the chemical structure of engine oil components as a result of operational aging processes. In the 1800 to 1475 cm−1 range, there are signals related to organic-oxygen products as well as oil nitration products, which are all acidic and thus reduce the alkaline reserve by reactions with additives increasing the alkaline reserve, which eventually leads to their depletion (an increase in the amount of oxidation products causes a decrease in the alkaline reserve). In the range of 1450 to 1000 cm−1, bands related to sulfur additives can be observed, those that improve lubricity, but also those that increase the alkaline reserve and disperse solids (an example of such an additive can be the basic calcium sulfonate). In this range, therefore, there are signals coming from sulfone additives, which gradually decrease during the operation of engine oil. Moreover, the bands of aromatic sulfonation products, which are formed during oil use, are also found in this range.
- Model C—the model was determined on the basis of mean absorbance values calculated in five wavenumber ranges, i.e., <1000 cm−1, 1000–1450 cm−1, 1475–1800 cm−1, 1800–2830 cm−1, and 2975–4000 cm−1. The indicated wavenumber ranges help identify any structural changes in the chemical structure of engine oil and the changes in background intensity. What is more, the fingerprint region of the spectrum below 1000 cm−1 was analyzed separately. As mentioned above, the calculations did not take into account the noise ranges occurring as a result of the generation of the differential spectrum.
- Model D—the model was based on the signal absorbance extremes at the wavenumbers corresponding to the molecular structures in chemical compounds that affect the base number value of the engine oil, i.e., 1746 cm−1 (carbonyls), 1631 cm−1 (nitro compounds), 1196 cm−1, 1169 cm−1, and 1062 cm−1 (sulfones and sulfonates).
- Model E—the model was based on the measurement of the surface areas of selected spectral bands, formed in the spectra as a result of vibrations of molecular structures in chemical compounds, affecting the value of the base number of engine oil (similarly to Model D), i.e.,1746 cm−1 (carbonyls), 1631 cm−1 (nitro compounds), 1196 cm−1, 1169 cm−1, and 1062 cm−1 (sulfones and sulfonates).
5. Discussions
6. Conclusions
7. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Parameter | Regression Coefficient |
|---|---|
| Intercept | 9.534 |
| ν <1000 | 233.191 |
| ν 1000–2000 | −122.828 |
| ν 2000–3000 | −156.564 |
| ν >3000 | 97.462 |
| Sample | Predictor Value | TBN (Measured) | TBN (Model Prediction) | |||
|---|---|---|---|---|---|---|
| ν <1000 | ν 1000–2000 | ν 2000–3000 | ν >3000 | |||
| SMP 1 | 0.0281 | 0.0584 | 0.0553 | 0.0775 | 8.18 | 7.81 |
| SMP 2 | 0.0417 | 0.0931 | 0.0838 | 0.1205 | 6.13 | 6.44 |
| SMP 3 | 0.0508 | 0.1142 | 0.1065 | 0.1531 | 5.24 | 5.61 |
| SMP 4 | 0.0629 | 0.1385 | 0.1298 | 0.1883 | 5.07 | 5.22 |
| SMP 5 | 0.0824 | 0.1776 | 0.1648 | 0.2388 | 4.67 | 4.41 |
| SMP 7 | 0.1100 | 0.2303 | 0.2175 | 0.3179 | 4.36 | 3.83 |
| SMP 8 | 0.1227 | 0.2562 | 0.2469 | 0.3610 | 2.85 | 3.21 |
| SMP 9 | 0.1361 | 0.2829 | 0.2743 | 0.4004 | 3.10 | 2.60 |
| SMP 10 | 0.1587 | 0.3201 | 0.3122 | 0.4540 | 2.13 | 2.58 |
| SMP 11 | 0.1688 | 0.3416 | 0.3326 | 0.4835 | 1.92 | 1.98 |
| SMP 12 | 0.1982 | 0.3922 | 0.3891 | 0.5617 | 1.67 | 1.39 |
| SMP 13 | 0.2138 | 0.4209 | 0.4185 | 0.6041 | 0.58 | 1.06 |
| SMP 14 | 0.2302 | 0.4518 | 0.4550 | 0.6543 | 0.50 | 0.26 |
| Parameter | Regression Coefficient |
|---|---|
| Intercept | 8.074 |
| ν 1000–1450 | −35.530 |
| ν 1475–1800 | 17.638 |
| Sample | Predictor Value | TBN (Measured) | TBN (Model Prediction) | |
|---|---|---|---|---|
| ν 1000–1450 | ν 1475–1800 | |||
| SMP 1 | 0.0598 | 0.0627 | 8.18 | 7.06 |
| SMP 2 | 0.0960 | 0.0996 | 6.13 | 6.42 |
| SMP 3 | 0.1177 | 0.1210 | 5.24 | 6.03 |
| SMP 4 | 0.1432 | 0.1450 | 5.07 | 5.54 |
| SMP 5 | 0.1855 | 0.1829 | 4.67 | 4.71 |
| SMP 7 | 0.2400 | 0.2377 | 4.36 | 3.74 |
| SMP 8 | 0.2666 | 0.2648 | 2.85 | 3.27 |
| SMP 9 | 0.2944 | 0.2932 | 3.10 | 2.78 |
| SMP 10 | 0.3289 | 0.3363 | 2.13 | 2.32 |
| SMP 11 | 0.3497 | 0.3618 | 1.92 | 2.03 |
| SMP 12 | 0.3983 | 0.4176 | 1.67 | 1.29 |
| SMP 13 | 0.4265 | 0.4489 | 0.58 | 0.84 |
| SMP 14 | 0.4563 | 0.4825 | 0.50 | 0.37 |
| Parameter | Regression Coefficient |
|---|---|
| Intercept | 9.839 |
| ν 1746 | 0.711 |
| ν 1631 | −4.814 |
| ν 1196 | −11.912 |
| ν 1169 | 1.568 |
| ν 1062 | −12.268 |
| Sample | Predictor Value (Based on the Area Peak) | TBN (Measured) | TBN (Model Prediction) | ||||
|---|---|---|---|---|---|---|---|
| ν 1746 | ν 1631 | ν 1196 | ν 1169 | ν 1062 | |||
| SMP 1 | 2.1780 | 0.5370 | 0.0830 | 0.2650 | 0.0410 | 8.18 | 7.73 |
| SMP 2 | 3.7280 | 0.5880 | 0.2900 | 0.5980 | 0.0680 | 6.13 | 6.31 |
| SMP 3 | 4.2960 | 0.6070 | 0.3990 | 0.7080 | 0.0500 | 5.24 | 5.72 |
| SMP 4 | 5.2430 | 0.6590 | 0.5320 | 0.8520 | 0.0320 | 5.07 | 5.00 |
| SMP 5 | 7.7870 | 0.8860 | 0.7410 | 1.4260 | 0.0170 | 4.67 | 4.31 |
| SMP 7 | 9.9400 | 1.3820 | 0.7280 | 1.8430 | 0.0370 | 4.36 | 4.02 |
| SMP 8 | 10.8930 | 1.6810 | 0.6760 | 1.9450 | 0.0490 | 2.85 | 3.89 |
| SMP 9 | 12.0260 | 2.1060 | 0.6640 | 2.1590 | 0.0630 | 3.10 | 2.96 |
| SMP 10 | 14.5990 | 2.4750 | 0.7480 | 2.4760 | 0.1010 | 2.13 | 2.04 |
| SMP 11 | 16.6630 | 2.6970 | 0.8130 | 2.7090 | 0.1330 | 1.92 | 1.64 |
| SMP 12 | 19.8570 | 2.9910 | 0.9050 | 2.9520 | 0.1670 | 1.67 | 1.36 |
| SMP 13 | 21.4380 | 3.3340 | 0.9070 | 3.0740 | 0.1750 | 0.58 | 0.91 |
| SMP 14 | 22.4560 | 3.5520 | 0.9110 | 3.1250 | 0.1840 | 0.50 | 0.50 |



References
- Khaziev, A.; Laushkin, A. Investigation of Changes in the Properties of Engine Oil Depending on the Sulfur Content in Gasoline. IOP Conf. Ser. Mater. Sci. Eng. 2020, 832, 012080. [Google Scholar] [CrossRef]
- Patel, N.; Shadangi, K.P. Characterization of Waste Engine Oil (WEO) Pyrolytic Oil and Diesel Blended Oil: Fuel Properties and Compositional Analysis. Mater. Today Proc. 2020, 33, 4933–4936. [Google Scholar] [CrossRef]
- Tayari, S.; Abedi, R.; Rahi, A. Comparative Assessment of Engine Performance and Emissions Fueled with Three Different Biodiesel Generations. Renew. Energy 2020, 147, 1058–1069. [Google Scholar] [CrossRef]
- Tayari, S.; Abedi, R.; Tahvildari, K. Experimental Investigation on Fuel Properties and Engine Characteristics of Biodiesel Produced from Eruca Sativa. SN Appl. Sci. 2020, 2, 2. [Google Scholar] [CrossRef] [Green Version]
- ASTM D8045-17e1; Standard Test Method for Acid Number of Crude Oils and Petroleum Products by Catalytic Thermometric Titration. ASTM International: West Conshohocken, PA, USA, 2017. [CrossRef]
- ASTM D974-14e2; Standard Test Method for Acid and Base Number by Color- Indicator Titration. ASTM International: West Conshohocken, PA, USA, 2014. [CrossRef]
- ASTM D664-18e2; Standard Test Method for Acid Number of Petroleum Products by Potentiometric Titration. ASTM International: West Conshohocken, PA, USA, 2018. [CrossRef]
- Wanke, P. In-Use Investigations of the Changes of Lubricant Properties in Diesel Engines. TEKA Comm. Mot. Energ. Agric. 2012, 12, 263–267. [Google Scholar]
- Masuko, M.; Ohkido, T.; Suzuki, A.; Ueno, T.; Okuda, S.; Sagawa, T. Fundamental Study of Changes in Friction and Wear Characteristics Due to ZnDTP Deterioration in Simulating Engine Oil Degradation during Use (Part 2)—Influences of the Presence of Peroxide and Dispersed Zn-Containing Solids. Dirac’s Differ. Equ. Phys. Finite Differ. 2005, 48, 769–777. [Google Scholar] [CrossRef]
- Vasanthan, B.; Devaradjane, G.; Shanmugam, V. Online Condition Monitoring of Lubricating Oil on Test Bench Diesel Engine & Vehicle. J. Chem. Pharm. Sci. 2015, 2015, 315–320. [Google Scholar]
- Kral, J.; Konecny, B.; Madac, K.; Fedorko, G.; Molnar, V. Degradation and Chemical Change of Longlife Oils Following Intensive Use in Automobile Engines. Measurement 2014, 50, 34–42. [Google Scholar] [CrossRef]
- Sharma, G.K.; Chawla, O.P. Modelling of Lubricant Oil Alkalinity in Diesel Engines. Tribol. Int. 1988, 21, 269–274. [Google Scholar] [CrossRef]
- Wolak, A. TBN Performance Study on a Test Fleet in Real-World Driving Conditions Using Present-Day Engine Oils. Measurement 2018, 114, 322–331. [Google Scholar] [CrossRef]
- Sentanuhady, J.; Majid, A.I.; Prashida, W.; Saputro, W.; Gunawan, N.P.; Raditya, T.Y.; Muflikhun, M.A. Analysis of the Effect of Biodiesel B20 and B100 on the Degradation of Viscosity and Total Base Number of Lubricating Oil in Diesel Engines with Long-Term Operation Using ASTM D2896 and ASTM D445-06 Methods. TEKNIK 2020, 41, 269–274. [Google Scholar] [CrossRef]
- Chikunova, A.S.; Vershinin, V.I. Determination of the Total Base Number of Engine Oils Using Potentiometric Titration. Ind. Lab. Diagn. Mater. 2020, 86, 5–12. [Google Scholar] [CrossRef]
- Growney, D.; Trickett, K.; Robin, M.; Rogers, S.; McDowall, D.; Moscrop, E. Acid Neutralization Rates—Why Total Base Number Doesn’t Tell the Whole Story: Understanding the Neutralization of Organic Acid in Engine Oils. SAE Int. J. Fuels Lubr. 2021, 14, 297. [Google Scholar] [CrossRef]
- Nagy, A.L.; Knaup, J.C.; Zsoldos, I. Investigation of Used Engine Oil Lubricating Performance Through Oil Analysis and Friction and Wear Measurements. Acta Tech. Jaurinensis 2019, 12, 237–251. [Google Scholar] [CrossRef] [Green Version]
- Wei, L.; Duan, H.; Jin, Y.; Jia, D.; Cheng, B.; Liu, J.; Li, J. Motor Oil Degradation during Urban Cycle Road Tests. Friction 2021, 9, 1002–1011. [Google Scholar] [CrossRef]
- Agocs, A.; Budnyk, S.; Frauscher, M.; Ronai, B.; Besser, C.; Dörr, N. Comparing Oil Condition in Diesel and Gasoline Engines. Ind. Lubr. Tribol. 2020, 72, 1033–1039. [Google Scholar] [CrossRef]
- Besser, C.; Agocs, A.; Ronai, B.; Ristic, A.; Repka, M.; Jankes, E.; McAleese, C.; Dörr, N. Generation of Engine Oils with Defined Degree of Degradation by Means of a Large Scale Artificial Alteration Method. Tribol. Int. 2019, 132, 39–49. [Google Scholar] [CrossRef]
- Kurre, S.K.; Pandey, S.; Khatri, N.; Bhurat, S.S.; Kumawat, S.K.; Saxena, S.; Kumar, S. Study of Lubricating Oil Degradation of Ci Engine Fueled with Diesel-Ethanol Blend. Tribol. Ind. 2021, 43, 222. [Google Scholar] [CrossRef]
- Robinson, N.; Hons, B.S. Monitoring Oil Degradation with Infrared Spectroscopy. Set Point Technol. 2000, 5, 1–8. [Google Scholar]
- Dyson, B.A.; Richards, L.J.; Eng, B.S.; Sc, W.; Williams, K.R.; Sc, B. Diesel Engine Lubricants: Their Selection and Utilization with Particular Reference to Oil Alkalinity. Ind. Lubr. Tribol. 1988, 9, 34–40. [Google Scholar] [CrossRef]
- Kauffman, R.E. Rapid, Portable Voltammetric Techniques for Performing Antioxidant, Total Acid Number (TAN) and Total Base Number (TBN) Measurements. Lubr. Eng. 1998, 54, 39–46. [Google Scholar]
- Bassbasi, M.; Hafid, A.; Platikanov, S.; Tauler, R.; Oussama, A. Study of Motor Oil Adulteration by Infrared Spectroscopy and Chemometrics Methods. Fuel 2013, 104, 798–804. [Google Scholar] [CrossRef]
- Agoston, A.; Schneidhofer, C.; Dörr, N.; Jakoby, B. A Concept of an Infrared Sensor System for Oil Condition Monitoring. Elektrotechnik Inf. 2008, 125, 71–75. [Google Scholar] [CrossRef]
- Barra, I.; Kharbach, M.; Qannari, E.M.; Hanafi, M.; Cherrah, Y.; Bouklouze, A. Predicting Cetane Number in Diesel Fuels Using FTIR Spectroscopy and PLS Regression. Vib. Spectrosc. 2020, 111, 103157. [Google Scholar] [CrossRef]
- Liu, Y.; Bao, K.; Wang, Q.; Zio, E. Application of FTIR Method to Monitor the Service Condition of Used Diesel Engine Lubricant Oil. In Proceedings of the 2019 4th International Conference on System Reliability and Safety, ICSRS 2019, Rome, Italy, 20–22 November 2019. [Google Scholar]
- Gracia, N.; Thomas, S.; Bazin, P.; Duponchel, L.; Thibault-Starzyk, F.; Lerasle, O. Combination of Mid-Infrared Spectroscopy and Chemometric Factorization Tools to Study the Oxidation of Lubricating Base Oils. Recent Dev. Operando Spectrosc. 2010, 155, 255–260. [Google Scholar] [CrossRef]
- Adams, M.J.; Romeo, M.J.; Rawson, P. FTIR Analysis and Monitoring of Synthetic Aviation Engine Oils. Talanta 2007, 73, 629–634. [Google Scholar] [CrossRef]
- Holland, T.; Abdul-Munaim, A.M.; Mandrell, C.; Karunanithy, R.; Watson, D.G.; Sivakumar, P. Uv-Visible Spectrophotometer for Distinguishing Oxidation Time of Engine Oil. Lubricants 2021, 9, 37. [Google Scholar] [CrossRef]
- Chimeno-Trinchet, C.; Murru, C.; Díaz-García, M.E.; Fernández-González, A.; Badía-Laíño, R. Artificial Intelligence and Fourier-Transform Infrared Spectroscopy for Evaluating Water-Mediated Degradation of Lubricant Oils. Talanta 2020, 219, 121312. [Google Scholar] [CrossRef]
- Nagy, A.L.; Agocs, A.; Ronai, B.; Raffai, P.; Rohde-Brandenburger, J.; Besser, C.; Dörr, N. Rapid Fleet Condition Analysis through Correlating Basic Vehicle Tracking Data with Engine Oil FT-IR Spectra. Lubricants 2021, 9, 114. [Google Scholar] [CrossRef]
- Wilson, D.; Allen, C. Experimental Validation of an Unscented Kalman Filter for Estimating Transient Engine Exhaust Composition with Fourier Transform Infrared Spectroscopy. Energy Fuels 2018, 32, 11899–11912. [Google Scholar] [CrossRef]
- Taghizadeh, A.; D’Souza, M. Quantification of Active Antioxidants by FTIR Spectroscopy and the Correlation to Measured TBN Values. SAE Tech. Pap. 2001, 110, 2072–2077. [Google Scholar] [CrossRef]
- Dong, J.; van de Voort, F.R.; Yaylayan, V.; Ismail, A.A.; Pinchuk, D.; Taghizadeh, A. Determination of Total Base Number (TBN) in Lubricating Oils by Mid-FTIR Spectroscopy. Lubr. Eng. 2001, 57, 24–30. [Google Scholar]
- Sejkorová, M.; Šarkan, B.; Veselík, P.; Hurtová, I. FTIR Spectrometry with PLS Regression for Rapid TBN Determination of Worn Mineral Engine Oils. Energies 2020, 13, 6438. [Google Scholar] [CrossRef]
- Macián, V.; Tormos, B.; García-Barberá, A.; Tsolakis, A. Applying Chemometric Procedures for Correlation the FTIR Spectroscopy with the New Thermometric Evaluation of Total Acid Number and Total Basic Number in Engine Oils. Chemom. Intell. Lab. Syst. 2021, 208, 104215. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.r-project.org/ (accessed on 1 March 2021).
- Agocs, A.; Besser, C.; Brenner, J.; Budnyk, S.; Frauscher, M.; Dörr, N. Engine Oils in the Field: A Comprehensive Tribological Assessment of Engine Oil Degradation in a Passenger Car. Tribol. Lett. 2022, 70, 28. [Google Scholar] [CrossRef]
- Sejkorová, M.; Kučera, M.; Hurtová, I.; Voltr, O. Application of FTIR-ATR Spectrometry in Conjunction with Multivariate Regression Methods for Viscosity Prediction of Worn-out Motor Oils. Appl. Sci. 2021, 11, 3842. [Google Scholar] [CrossRef]








| The Parameter | The Value |
|---|---|
| Maximum torque | 340 Nm at 2000 rpm |
| Forced induction | Turbocharger (gas compressor) |
| Number of cylinders | 4 |
| Cylinder arrangement | Straight (inline) |
| Number of valves | 16 |
| Injection type | Common Rail |
| Lubrication system capacity | 5.5 L |
| Manual gearbox | 6-gear |
| Transmission type | Rear axle |
| Sample Code | Number of In-Service Days | Sampling Date (D-M-Y) | Mileage [km] |
|---|---|---|---|
| SMP 0 | 0 | 17.7.2019 | 0 |
| SMP 1 | 0 | 17.7.2019 | 13 |
| SMP 2 | 28 | 15.8.2019 | 1157 |
| SMP 3 | 42 | 29.8.2019 | 2175 |
| SMP 4 | 55 | 12.9.2019 | 3116 |
| SMP 5 | 105 | 2.11.2019 | 4220 |
| SMP 6 | 150 | 17.12.2019 | 5265 |
| SMP 7 | 170 | 7.1.2020 | 6332 |
| SMP 8 | 208 | 15.2.2020 | 7319 |
| SMP 9 | 290 | 7.5.2020 | 8498 |
| SMP 10 | 332 | 19.6.2020 | 9811 |
| SMP 11 | 354 | 11.7.2020 | 11,021 |
| SMP 12 | 409 | 6.9.2020 | 12,460 |
| SMP 13 | 436 | 3.10.2020 | 13,521 |
| SMP 14 | 470 | 7.11.2020 | 14,820 |
| Sample Code | TBN | A at 1746 cm−1 | A at 1631 cm−1 | A at 1169 cm−1 |
| SMP 1 * | 8.18 mg KOH/g | 0.131 | 0.109 | 0.087 |
| Relative Change | ||||
| SMP 2 | −25% | 111% | 46% | 76% |
| SMP 3 | −15% | 23% | 18% | 22% |
| SMP 4 | −3% | 23% | 17% | 22% |
| SMP 5 | −8% | 42% | 23% | 38% |
| SMP 7 | −7% | 4% | 13% | 8% |
| SMP 8 | −35% | 4% | 11% | 8% |
| SMP 9 | 9% | 4% | 12% | 9% |
| SMP 10 | −31% | 15% | 15% | 14% |
| SMP 11 | −10% | 10% | 8% | 8% |
| SMP 12 | −13% | 14% | 13% | 13% |
| SMP 13 | −65% | 5% | 8% | 6% |
| SMP 14 | −14% | 4% | 7% | 5% |
| Parameter | Regression Coefficient |
|---|---|
| Intercept | 9.682 |
| ν <1000 | 316.270 |
| ν 1000–1450 | −10.326 |
| ν 1475–1800 | −133.000 |
| ν 1800–2830 | −36.081 |
| ν 2975–4000 | 3.841 |
| Sample | Predictor Value | TBN (Measured) | TBN (Model Prediction) | ||||
|---|---|---|---|---|---|---|---|
| ν <1000 | ν 1000–1450 | ν 1475–1800 | ν 1800–2830 | ν 2975–4000 | |||
| SMP 1 | 0.0281 | 0.0598 | 0.0627 | 0.0524 | 0.0771 | 8.18 | 8.03 |
| SMP 2 | 0.0417 | 0.0960 | 0.0996 | 0.0800 | 0.1196 | 6.13 | 6.20 |
| SMP 3 | 0.0508 | 0.1177 | 0.1210 | 0.1010 | 0.1524 | 5.24 | 5.40 |
| SMP 4 | 0.0629 | 0.1432 | 0.1450 | 0.1243 | 0.1870 | 5.07 | 5.04 |
| SMP 5 | 0.0824 | 0.1855 | 0.1829 | 0.1582 | 0.2371 | 4.67 | 4.69 |
| SMP 7 | 0.1100 | 0.2400 | 0.2377 | 0.2102 | 0.3152 | 4.36 | 4.00 |
| SMP 8 | 0.1227 | 0.2666 | 0.2648 | 0.2377 | 0.3583 | 2.85 | 3.33 |
| SMP 9 | 0.1361 | 0.2944 | 0.2932 | 0.2629 | 0.3979 | 3.10 | 2.74 |
| SMP 10 | 0.1587 | 0.3289 | 0.3363 | 0.3007 | 0.4507 | 2.13 | 2.63 |
| SMP 11 | 0.1688 | 0.3497 | 0.3618 | 0.3196 | 0.4804 | 1.92 | 1.66 |
| SMP 12 | 0.1982 | 0.3983 | 0.4176 | 0.3736 | 0.5583 | 1.67 | 1.37 |
| SMP 13 | 0.2138 | 0.4265 | 0.4489 | 0.4022 | 0.6004 | 0.58 | 0.99 |
| SMP 14 | 0.2302 | 0.4563 | 0.4825 | 0.4375 | 0.6503 | 0.50 | 0.32 |
| Parameter | Regression Coefficient |
|---|---|
| Intercept | 9.033 |
| ν 1746 | 38.416 |
| ν 1631 | 14.971 |
| ν 1196 | −287.984 |
| ν 1169 | 82.665 |
| ν 1062 | 154.500 |
| Sample | Predictor Value (Based on the Signal Peak) | TBN (Measured) | TBN (Model Prediction) | ||||
|---|---|---|---|---|---|---|---|
| ν 1746 | ν 1631 | ν 1196 | ν 1169 | ν 1062 | |||
| SMP 1 | 0.1314 | 0.1090 | 0.0830 | 0.0869 | 0.0575 | 8.18 | 7.87 |
| SMP 2 | 0.2766 | 0.1589 | 0.1466 | 0.1532 | 0.0902 | 6.13 | 6.41 |
| SMP 3 | 0.3400 | 0.1871 | 0.1801 | 0.1863 | 0.1114 | 5.24 | 5.64 |
| SMP 4 | 0.4183 | 0.2180 | 0.2206 | 0.2269 | 0.1367 | 5.07 | 4.72 |
| SMP 5 | 0.5922 | 0.2680 | 0.2924 | 0.3135 | 0.1748 | 4.67 | 4.51 |
| SMP 7 | 0.7019 | 0.3461 | 0.3595 | 0.3883 | 0.2231 | 4.36 | 4.23 |
| SMP 8 | 0.7274 | 0.3859 | 0.3877 | 0.4180 | 0.2448 | 2.85 | 3.49 |
| SMP 9 | 0.7588 | 0.4338 | 0.4193 | 0.4537 | 0.2672 | 3.10 | 2.70 |
| SMP 10 | 0.8730 | 0.4981 | 0.4745 | 0.5163 | 0.2994 | 2.13 | 2.32 |
| SMP 11 | 0.9591 | 0.5381 | 0.5099 | 0.5566 | 0.3162 | 1.92 | 1.95 |
| SMP 12 | 1.0948 | 0.6098 | 0.5774 | 0.6275 | 0.3590 | 1.67 | 1.28 |
| SMP 13 | 1.1452 | 0.6605 | 0.6120 | 0.6652 | 0.3829 | 0.58 | 0.80 |
| SMP 14 | 1.1873 | 0.7054 | 0.6440 | 0.6968 | 0.4087 | 0.50 | 0.48 |
| Model Fit Statistics | Model A | Model B | Model C | Model D | Model E |
|---|---|---|---|---|---|
| R2 | 0.973 | 0.950 | 0.982 | 0.980 | 0.966 |
| Adjusted R2 | 0.959 | 0.940 | 0.968 | 0.964 | 0.942 |
| Root mean square error | 0.460 | 0.560 | 0.405 | 0.431 | 0.548 |
| Model A | Model B | Model C | Model D | Model E | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 * | 2 * | 1 * | 2 * | 1 * | 2 * | 1 * | 2 * | 1 * | 2 * | |
| SMP 1 | 0.37 | 5% | 1.12 | 14% | 0.15 | 2% | 0.31 | 4% | 0.45 | 6% |
| SMP 2 | 0.31 | 5% | 0.29 | 5% | 0.07 | 1% | 0.28 | 5% | 0.18 | 3% |
| SMP 3 | 0.37 | 7% | 0.79 | 15% | 0.16 | 3% | 0.40 | 8% | 0.48 | 9% |
| SMP 4 | 0.15 | 3% | 0.47 | 9% | 0.03 | 1% | 0.35 | 7% | 0.07 | 1% |
| SMP 5 | 0.26 | 6% | 0.04 | 1% | 0.02 | 1% | 0.16 | 3% | 0.36 | 8% |
| SMP 7 | 0.53 | 12% | 0.62 | 14% | 0.36 | 8% | 0.13 | 3% | 0.34 | 8% |
| SMP 8 | 0.36 | 13% | 0.42 | 15% | 0.48 | 17% | 0.64 | 22% | 1.04 | 37% |
| SMP 9 | 0.50 | 16% | 0.32 | 10% | 0.36 | 12% | 0.40 | 13% | 0.14 | 5% |
| SMP 10 | 0.45 | 21% | 0.19 | 9% | 0.50 | 23% | 0.19 | 9% | 0.09 | 4% |
| SMP 11 | 0.06 | 3% | 0.11 | 6% | 0.26 | 13% | 0.03 | 2% | 0.28 | 15% |
| SMP 12 | 0.28 | 17% | 0.38 | 23% | 0.30 | 18% | 0.39 | 23% | 0.31 | 18% |
| SMP 13 | 0.48 | 82% | 0.26 | 44% | 0.41 | 72% | 0.22 | 38% | 0.33 | 57% |
| SMP 14 | 0.24 | 49% | 0.13 | 26% | 0.18 | 36% | 0.02 | 4% | 0.00 | 1% |
| mean | 0.34 | 18% | 0.40 | 15% | 0.25 | 16% | 0.27 | 11% | 0.31 | 13% |
| SMP 1–7 | 0.33 | 6% | 0.33 | 10% | 0.26 | 3% | 0.27 | 5% | 0.30 | 6% |
| SMP 8–14 | 0.33 | 29% | 0.34 | 19% | 0.28 | 27% | 0.27 | 16% | 0.31 | 19% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolak, A.; Molenda, J.; Fijorek, K.; Łankiewicz, B. Prediction of the Total Base Number (TBN) of Engine Oil by Means of FTIR Spectroscopy. Energies 2022, 15, 2809. https://doi.org/10.3390/en15082809
Wolak A, Molenda J, Fijorek K, Łankiewicz B. Prediction of the Total Base Number (TBN) of Engine Oil by Means of FTIR Spectroscopy. Energies. 2022; 15(8):2809. https://doi.org/10.3390/en15082809
Chicago/Turabian StyleWolak, Artur, Jarosław Molenda, Kamil Fijorek, and Bartosz Łankiewicz. 2022. "Prediction of the Total Base Number (TBN) of Engine Oil by Means of FTIR Spectroscopy" Energies 15, no. 8: 2809. https://doi.org/10.3390/en15082809
APA StyleWolak, A., Molenda, J., Fijorek, K., & Łankiewicz, B. (2022). Prediction of the Total Base Number (TBN) of Engine Oil by Means of FTIR Spectroscopy. Energies, 15(8), 2809. https://doi.org/10.3390/en15082809