Denitrification in Microbial Fuel Cells Using Granular Activated Carbon as an Effective Biocathode
Abstract
:1. Introduction
2. Materials and Methods
2.1. MFC Configuration
2.2. Inoculum and Operating Conditions
2.3. Enrichment of Bacteria on GAC
2.4. MFC Operation with Enriched GAC
2.5. Electrochemical Analysis and Calculation
2.6. Scanning Electron Microscope
2.7. Nitrate Analysis
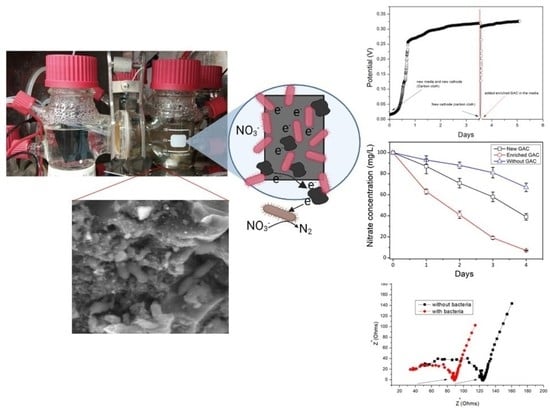
3. Results
3.1. Denitrifying Bacteria on GAC
3.2. NO3−-N Removal in the MFC Using the GAC-Biocathode
3.3. Effect of GAC (Enriched and New GAC) in the Cathode Chamber
3.4. Electrochemical Properties of the Biocathode
3.5. SEM Images of Bacteria Grown on Enriched GAC on the Biocathode
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, G.; Wang, J.; Liu, L.; Li, Y.; Zhang, Y.; Wang, S. The Analysis of Groundwater Nitrate Pollution and Health Risk Assessment in Rural Areas of Yantai, China. BMC Public Health 2020, 20, 437. [Google Scholar] [CrossRef] [PubMed]
- Vijay, A.; Chhabra, M.; Vincent, T. Microbial Community Modulates Electrochemical Performance and Denitrification Rate in a Biocathodic Autotrophic and Heterotrophic Denitrifying Microbial Fuel Cell. Bioresour. Technol. 2019, 272, 217–225. [Google Scholar] [CrossRef]
- Bijay-Singh; Craswell, E. Fertilizers and Nitrate Pollution of Surface and Ground Water: An Increasingly Pervasive Global Problem. SN Appl. Sci. 2021, 3, 518. [Google Scholar] [CrossRef]
- Naga Samrat, M.V.V.; Kesava Rao, K.; Ruggeri, B.; Tommasi, T. Denitrification of Water in a Microbial Fuel Cell (MFC) Using Seawater Bacteria. J. Clean. Prod. 2018, 178, 449–456. [Google Scholar] [CrossRef]
- Rayaroth, M.P.; Aravindakumar, C.T.; Shah, N.S.; Boczkaj, G. Advanced Oxidation Processes (AOPs) Based Wastewater Treatment—Unexpected Nitration Side Reactions—A Serious Environmental Issue: A Review. Chem. Eng. J. 2022, 430, 133002. [Google Scholar] [CrossRef]
- Lin, L.; Luo, Z.; Ishida, K.; Urasaki, K.; Kubota, K.; Li, Y.Y. Fast Formation of Anammox Granules Using a Nitrification-Denitrification Sludge and Transformation of Microbial Community. Water Res. 2022, 221, 118751. [Google Scholar] [CrossRef]
- Hao, Z.L.; Ali, A.; Ren, Y.; Su, J.F.; Wang, Z. A Mechanistic Review on Aerobic Denitrification for Nitrogen Removal in Water Treatment. Sci. Total Environ. 2022, 847, 157452. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Ying, X.-B.; Zhao, N.-N.; Yu, S.-S.; Zhang, X.; Feng, H.-J.; Zhang, Y.-F.; Yu, H.-Q. Interspecies Electron Transfer between Geobacter and Denitrifying Bacteria for Nitrogen Removal in Bioelectrochemical System. Chem. Eng. J. 2022, 455, 139821. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, F.; He, W.; Zhang, X.; Feng, Y.; Logan, B.E. Intermittent Contact of Fluidized Anode Particles Containing Exoelectrogenic Biofilms for Continuous Power Generation in Microbial Fuel Cells. J. Power Sources 2014, 261, 278–284. [Google Scholar] [CrossRef]
- Li, R.; Feng, C.; Hu, W.; Xi, B.; Chen, N.; Zhao, B.; Liu, Y.; Hao, C.; Pu, J. Woodchip-Sulfur Based Heterotrophic and Autotrophic Denitrification (WSHAD) Process for Nitrate Contaminated Water Remediation. Water Res. 2016, 89, 171–179. [Google Scholar] [CrossRef]
- Di Capua, F.; Pirozzi, F.; Lens, P.N.L.; Esposito, G. Electron Donors for Autotrophic Denitrification. Chem. Eng. J. 2019, 362, 922–937. [Google Scholar] [CrossRef]
- Nguyen, V.K.; Hong, S.; Park, Y.; Jo, K.; Lee, T. Autotrophic Denitrification Performance and Bacterial Community at Biocathodes of Bioelectrochemical Systems with Either Abiotic or Biotic Anodes. J. Biosci. Bioeng. 2015, 119, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.; Baek, G.; Logan, B.E. Vapor-Fed Cathode Microbial Electrolysis Cells with Closely Spaced Electrodes Enables Greatly Improved Performance. Environ. Sci. Technol. 2022, 56, 1211–1220. [Google Scholar] [CrossRef] [PubMed]
- Ki, D.; Popat, S.C.; Torres, C.I. Reduced Overpotentials in Microbial Electrolysis Cells through Improved Design, Operation, and Electrochemical Characterization. Chem. Eng. J. 2016, 287, 181–188. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Sun, X.; Yin, D.; Cai, L.; Zhang, L. Suspended Anode-Type Microbial Fuel Cells for Enhanced Electricity Generation. RSC Adv. 2020, 10, 9868–9877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Q.; Zhao, H.; Liang, H.H. Denitrification of Overlying Water by Microbial Electrochemical Snorkel. Bioresour. Technol. 2015, 197, 512–514. [Google Scholar] [CrossRef]
- Caizán-Juanarena, L.; Sleutels, T.; Borsje, C.; ter Heijne, A. Considerations for Application of Granular Activated Carbon as Capacitive Bioanode in Bioelectrochemical Systems. Renew. Energy 2020, 157, 782–792. [Google Scholar] [CrossRef]
- Jayalakshmi, M.; Balasubramanian, K. Simple Capacitors to Supercapacitors—An Overview. Int. J. Electrochem. Sci. 2008, 3, 1196–1217. [Google Scholar]
- Sharma, P.; Bhatti, T.S. A Review on Electrochemical Double-Layer Capacitors. Energy Convers. Manag. 2010, 51, 2901–2912. [Google Scholar] [CrossRef]
- Wu, Y.; Guan, K.; Wang, Z.; Xu, B.; Zhao, F. Isolation, Identification and Characterization of an Electrogenic Microalgae Strain. PLoS ONE 2013, 8, e73442. [Google Scholar] [CrossRef]
- Tursun, H.; Liu, R.; Li, J.; Abro, R.; Wang, X.; Gao, Y.; Li, Y. Carbon Material Optimized Biocathode for Improving Microbial Fuel Cell Performance. Front. Microbiol. 2016, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Deeke, A.; Sleutels, T.H.J.A.; Donkers, T.F.W.; Hamelers, H.V.M.; Buisman, C.J.N.; Ter Heijne, A. Fluidized Capacitive Bioanode as a Novel Reactor Concept for the Microbial Fuel Cell. Environ. Sci. Technol. 2015, 49, 1929–1935. [Google Scholar] [CrossRef] [PubMed]
- Frackowiak, E.; Béguin, F. Carbon Materials for the Electrochemical Storage of Energy in Capacitors. Carbon 2001, 39, 937–950. [Google Scholar] [CrossRef]
- Borsje, C.; Liu, D.; Sleutels, T.H.J.A.; Buisman, C.J.N.; ter Heijne, A. Performance of Single Carbon Granules as Perspective for Larger Scale Capacitive Bioanodes. J. Power Sources 2016, 325, 690–696. [Google Scholar] [CrossRef] [Green Version]
- Matsena, M.T.; Mabuse, M.; Tichapondwa, S.M.; Chirwa, E.M.N. Improved Performance and Cost Efficiency by Surface Area Optimization of Granular Activated Carbon in Air-Cathode Microbial Fuel Cell. Chemosphere 2021, 281, 130941. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gurung, A.; Thapa, B.S.; Ko, S.-Y.; Ashun, E.; Toor, U.A.; Oh, S.-E. Denitrification in Microbial Fuel Cells Using Granular Activated Carbon as an Effective Biocathode. Energies 2023, 16, 709. https://doi.org/10.3390/en16020709
Gurung A, Thapa BS, Ko S-Y, Ashun E, Toor UA, Oh S-E. Denitrification in Microbial Fuel Cells Using Granular Activated Carbon as an Effective Biocathode. Energies. 2023; 16(2):709. https://doi.org/10.3390/en16020709
Chicago/Turabian StyleGurung, Anup, Bhim Sen Thapa, Seong-Yun Ko, Ebenezer Ashun, Umair Ali Toor, and Sang-Eun Oh. 2023. "Denitrification in Microbial Fuel Cells Using Granular Activated Carbon as an Effective Biocathode" Energies 16, no. 2: 709. https://doi.org/10.3390/en16020709
APA StyleGurung, A., Thapa, B. S., Ko, S.-Y., Ashun, E., Toor, U. A., & Oh, S.-E. (2023). Denitrification in Microbial Fuel Cells Using Granular Activated Carbon as an Effective Biocathode. Energies, 16(2), 709. https://doi.org/10.3390/en16020709