Biomass Polygeneration System for the Thermal Conversion of Softwood Waste into Hydrogen and Drop-In Biofuels
Abstract
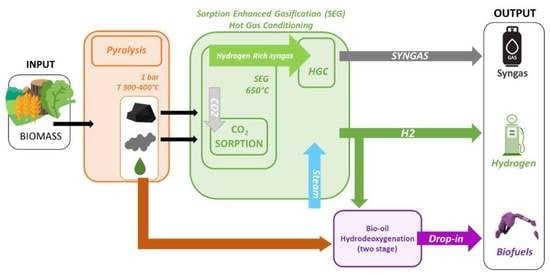
:1. Introduction
2. Materials and Methods
2.1. Feedstock
2.2. Pyrolysis
Pyrolysis Validation
2.3. Gasifier
Gasification Validation
2.4. Gas Cleaning
2.5. Hydrodeoxygenation
2.5.1. Hydrotreatment
2.5.2. Distillation
2.5.3. Hydrocracking
3. Results and Discussion
- Four pyrolysis conditions: no pyrolysis, Tpyro = 300 °C, Tpyro = 350 °C, Tpyro = 400 °C;
- Five CaO/char ratios: from 1 to 2 by step of 0.25;
- Ten steam/char ratios: from 0.2 to 2 in 0.2 steps.
4. Conclusions
- The results of the analysis of the sensitivity of the steam/char, CaO/char and pyrolysis temperature showed that the pyrolysis pretreatment provides noteworthy advantages, including an increase in the overall energy efficiency (up to a pyrolysis temperature of 400 °C) and a diversification of the energy outputs, since a drop-in fuel with high heating value was obtained by upgrading the pyrolysis bio-oil;
- A correlation between the pyrolysis temperature and the steam/char ratio was found to influence the overall peak efficiency value. In particular, a set of optimal operating parameters toward energy efficiency is Tpyro = 400 °C, Steam/Char = 1.6 and CaO/Char = 1.5. At such optimal values, an overall efficiency of about 75% is obtained, with hydrogen, syngas, and drop-in mass yields of 2%, 34% and 26%;
- The maximum total output energy flux is obtained at TPyro 350 °C while hydrogen production capacity is maximized with reduced TPyro, with a peak without pyrolysis pretreatment at 8% hydrogen mass yield and 10 MJ/kgbiomass energy yield;
- The system can be designed and operated flexibly according to different pyrolysis operating temperatures to maximize the production capacity of either biofuel or hydrogen or to maximize the energy efficiency of the overall process.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- United Nations Environment Programme. Emissions Gap Report 2022: The Closing Window—Climate Crisis Calls for Rapid Transformation of Societies. Nairobi. 2022. Volume 20, No. 2. Available online: https://www.unep.org/emissions-gap-report-2022 (accessed on 27 October 2022).
- IEA. World Energy Outlook 2021; IEA Publications: Paris, France, 2021; p. 15. Available online: www.iea.org/weo (accessed on 1 October 2021).
- EU. Directive (EU) 2018/2001 of the European Parliament and of the Council on the Promotion of the Use of Energy from Renewable Sources. Off. J. Eur. Union 2018, L328, 82–209. [Google Scholar]
- Erbach, G.; Jensen, L.; European Parliament. Briefing towards Climate Neutrality: Fit for 55 Packagel; European Parliamentary Research Service: Brussels, Belgium, 2022.
- Soone, J. Deployment of Alternative Fuels Infrastructure: Fit for 55 Package; European Parliamentary Research Service: Brussels, Belgium, 2022.
- Soone, J. ReFuelEU Aviation Initiative: Sustainable Aviation Fuels and the Fit for 55 Package; Think Tank|European Parliament: Brussels, Belgium, 2022. [Google Scholar]
- Maritime, F. EU Legislation in Progress Sustainable Maritime Fuels ‘Fit for 55’ Package: The FuelEU Maritime Proposal; European Parliamentary Research Service: Brussels, Belgium, 2021.
- dan Rosad, S. Proposal for a Regulation of the European Parliament and of the Council on ensuring a level playing field for sustainable air transport—‘ReFuelEU Aviation’. Suparyanto Dan Rosad 2015, 5, 248–253. [Google Scholar]
- Nadel, S. Electrification in the Transportation, Buildings, and Industrial Sectors: A Review of Opportunities, Barriers, and Policies. Curr. Sustain. Energy Rep. 2019, 6, 158–168. [Google Scholar] [CrossRef]
- Panoutsou, C.; Giarola, S.; Ibrahim, D.; Verzandvoort, S.; Elbersen, B.; Sandford, C.; Malins, C.; Politi, M.; Vourliotakis, G.; Zita, V.E.; et al. Opportunities for Low Indirect Land Use Biomass for Biofuels in Europe. Appl. Sci. 2022, 12, 4623. [Google Scholar] [CrossRef]
- Bashir, M.A.; Lima, S.; Jahangiri, H.; Majewski, A.J.; Hofmann, M.; Hornung, A.; Ouadi, M. A step change towards sustainable aviation fuel from sewage sludge. J. Anal. Appl. Pyrolysis 2022, 163, 105498. [Google Scholar] [CrossRef]
- Chen, Y.-K.; Lin, C.-H.; Wang, W.-C. The conversion of biomass into renewable jet fuel. Energy 2020, 201, 117655. [Google Scholar] [CrossRef]
- Dossow, M.; Dieterich, V.; Hanel, A.; Spliethoff, H.; Fendt, S. Improving carbon efficiency for an advanced Biomass-to-Liquid process using hydrogen and oxygen from electrolysis. Renew. Sustain. Energy Rev. 2021, 152, 111670. [Google Scholar] [CrossRef]
- Díaz-Pérez, M.A.; Serrano-Ruiz, J.C. Catalytic Production of Jet Fuels from Biomass. Molecules 2020, 25, 802. [Google Scholar] [CrossRef] [Green Version]
- Elkasabi, Y.; Mullen, C.A.; Boateng, A.A.; Brown, A.; Timko, M.T. Flash Distillation of Bio-Oils for Simultaneous Production of Hydrocarbons and Green Coke. Ind. Eng. Chem. Res. 2019, 58, 1794–1802. [Google Scholar] [CrossRef]
- Lahijani, P.; Mohammadi, M.; Mohamed, A.R.; Ismail, F.; Lee, K.T.; Amini, G. Upgrading biomass-derived pyrolysis bio-oil to bio-jet fuel through catalytic cracking and hydrodeoxygenation: A review of recent progress. Energy Convers. Manag. 2022, 268, 115956. [Google Scholar] [CrossRef]
- Nguyen, T.-V.; Clausen, L.R. Thermodynamic analysis of polygeneration systems based on catalytic hydropyrolysis for the production of bio-oil and fuels. Energy Convers. Manag. 2018, 171, 1617–1638. [Google Scholar] [CrossRef]
- Marcantonio, V.; Ferrario, A.M.; Di Carlo, A.; Del Zotto, L.; Monarca, D.; Bocci, E. Biomass Steam Gasification: A Comparison of Syngas Composition between a 1-D MATLAB Kinetic Model and a 0-D Aspen Plus Quasi-Equilibrium Model. Computation 2020, 8, 86. [Google Scholar] [CrossRef]
- Schmid, J.C.; Fuchs, J.; Benedikt, F.; Mauerhofer, A.M.; Müller, S.; Hofbauer, H.; Stocker, H.; Kieberge, N.; Bürgler, T. Sorption enhanced reforming with the novel dual fluidized bed test plant at tu wien. In Proceedings of the European Biomass Conference and Exhibition (EUBCE), Stockholm, Sweden, 12–15 June 2017. [Google Scholar]
- Marcantonio, V.; De Falco, M.; Capocelli, M.; Bocci, E.; Colantoni, A.; Villarini, M. Process analysis of hydrogen production from biomass gasification in fluidized bed reactor with different separation systems. Int. J. Hydrogen Energy 2019, 44, 10350–10360. [Google Scholar] [CrossRef]
- Marcantonio, V.; Müller, M.; Bocci, E. A Review of Hot Gas Cleaning Techniques for Hydrogen Chloride Removal from Biomass-Derived Syngas. Energies 2021, 14, 6519. [Google Scholar] [CrossRef]
- Marcantonio, V.; Bocci, E.; Ouweltjes, J.P.; Del Zotto, L.; Monarca, D. Evaluation of sorbents for high temperature removal of tars, hydrogen sulphide, hydrogen chloride and ammonia from biomass-derived syngas by using Aspen Plus. Int. J. Hydrogen Energy 2020, 45, 6651–6662. [Google Scholar] [CrossRef]
- Gottschalk, F.; La Rosa, M. Process Configuration. In Modern Business Process Automation; Springer: Berlin/Heidelberg, Germany, 2009; pp. 459–487. [Google Scholar] [CrossRef]
- Prestipino, M.; Chiodo, V.; Maisano, S.; Zafarana, G.; Urbani, F.; Galvagno, A. Hydrogen rich syngas production by air-steam gasification of citrus peel residues from citrus juice manufacturing: Experimental and simulation activities. Int. J. Hydrogen Energy 2017, 42, 26816–26827. [Google Scholar] [CrossRef]
- Bach, Q.-V.; Nguyen, D.D.; Lee, C.-J. Effect of Torrefaction on Steam Gasification of Biomass in Dual Fluidized Bed Reactor—A Process Simulation Study. BioEnergy Res. 2019, 12, 1042–1051. [Google Scholar] [CrossRef]
- Treusch, K.; Mauerhofer, A.M.; Schwaiger, N.; Pucher, P.; Müller, S.; Painer, D.; Hofbauer, H.; Siebenhofer, M. Hydrocarbon production by continuous hydrodeoxygenation of liquid phase pyrolysis oil with biogenous hydrogen rich synthesis gas. React. Chem. Eng. 2019, 4, 1195–1207. [Google Scholar] [CrossRef] [Green Version]
- Doelle, K.; Bajrami, B. Sodium Hydroxide and Calcium Hydroxide Hybrid Oxygen Bleaching with System. IOP Conf. Series Mater. Sci. Eng. 2018, 301, 012136. [Google Scholar] [CrossRef]
- Ranzi, E.; Cuoci, A.; Faravelli, T.; Frassoldati, A.; Migliavacca, G.; Pierucci, S.; Sommariva, S. Chemical Kinetics of Biomass Pyrolysis. Energy Fuels 2008, 22, 4292–4300. [Google Scholar] [CrossRef]
- Debiagi, P.; Gentile, G.; Cuoci, A.; Frassoldati, A.; Ranzi, E.; Faravelli, T. A predictive model of biochar formation and characterization. J. Anal. Appl. Pyrolysis 2018, 134, 326–335. [Google Scholar] [CrossRef]
- Oyedun, A.; Patel, M.; Kumar, M.; Kumar, A. The Upgrading of Bio-Oil via Hydrodeoxygenation. In Chemical Catalysts for Biomass Upgrading; Wiley: New York, NY, USA, 2019. [Google Scholar]
- Lazzari, E.; Schena, T.; Marcelo, M.C.A.; Primaz, C.T.; Silva, A.N.; Ferrão, M.F.; Bjerk, T.; Caramão, E.B. Classification of biomass through their pyrolytic bio-oil composition using FTIR and PCA analysis. Ind. Crops Prod. 2018, 111, 856–864. [Google Scholar] [CrossRef]
- Elliott, D.C.; Meier, D.; Oasmaa, A.; van de Beld, B.; Bridgwater, A.V.; Marklund, M. Results of the International Energy Agency Round Robin on Fast Pyrolysis Bio-oil Production. Energy Fuels 2017, 31, 5111–5119. [Google Scholar] [CrossRef] [Green Version]
- Zadeh, Z.E.; Abdulkhani, A.; Saha, B. Characterization of Fast Pyrolysis Bio-Oil from Hardwood and Softwood Lignin. Energies 2020, 13, 887. [Google Scholar] [CrossRef] [Green Version]
- Liaw, S.-S.; Wang, Z.; Ndegwa, P.; Frear, C.; Ha, S.; Li, C.-Z.; Garcia-Perez, M. Effect of pyrolysis temperature on the yield and properties of bio-oils obtained from the auger pyrolysis of Douglas Fir wood. J. Anal. Appl. Pyrolysis 2012, 93, 52–62. [Google Scholar] [CrossRef]
- Cao, X.; Pignatello, J.J.; Li, Y.; Lattao, C.; Chappell, M.A.; Chen, N.; Miller, L.F.; Mao, J. Characterization of Wood Chars Produced at Different Temperatures Using Advanced Solid-State 13C NMR Spectroscopic Techniques. Energy Fuels 2012, 26, 5983–5991. [Google Scholar] [CrossRef]
- Roger, R.P.M.A.T.; Rowell, M. Handbook of Wood Chemistry and Wood Composites; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Barisano, D.; Canneto, G.; Nanna, F.; Villone, A.; Fanelli, E.; Freda, C.; Grieco, M.; Cornacchia, G.; Braccio, G.; Marcantonio, V.; et al. Investigation of an Intensified Thermo-Chemical Experimental Set-Up for Hydrogen Production from Biomass: Gasification Process Performance—Part I. Processes 2021, 9, 1104. [Google Scholar] [CrossRef]
- Fermoso, J.; Rubiera, F.; Chen, D. Sorption enhanced catalytic steam gasification process: A direct route from lignocellulosic biomass to high purity hydrogen. Energy Environ. Sci. 2012, 5, 6358–6367. [Google Scholar] [CrossRef]
- Zhai, M.; Guo, L.; Wang, Y.; Zhang, Y.; Dong, P.; Jin, H. Process simulation of staging pyrolysis and steam gasification for pine sawdust. Int. J. Hydrogen Energy 2016, 41, 21926–21935. [Google Scholar] [CrossRef]
- Puig-Gamero, M.; Argudo-Santamaria, J.; Valverde, J.; Sánchez, P.; Sanchez-Silva, L. Three integrated process simulation using aspen plus®: Pine gasification, syngas cleaning and methanol synthesis. Energy Convers. Manag. 2018, 177, 416–427. [Google Scholar] [CrossRef]
- Begum, S.; Rasul, M.G.; Akbar, D.; Ramzan, N. Performance Analysis of an Integrated Fixed Bed Gasifier Model for Different Biomass Feedstocks. Energies 2013, 6, 6508–6524. [Google Scholar] [CrossRef]
- Marcantonio, V.; Bocci, E.; Monarca, D. Development of a Chemical Quasi-Equilibrium Model of Biomass Waste Gasification in a Fluidized-Bed Reactor by Using Aspen Plus. Energies 2019, 13, 53. [Google Scholar] [CrossRef] [Green Version]
- Savuto, E.; Di Carlo, A.; Steele, A.; Heidenreich, S.; Gallucci, K.; Rapagnà, S. Syngas conditioning by ceramic filter candles filled with catalyst pellets and placed inside the freeboard of a fluidized bed steam gasifier. Fuel Process. Technol. 2019, 191, 44–53. [Google Scholar] [CrossRef]
- Moradi, R.; Marcantonio, V.; Cioccolanti, L.; Bocci, E. Integrating biomass gasification with a steam-injected micro gas turbine and an Organic Rankine Cycle unit for combined heat and power production. Energy Convers. Manag. 2020, 205, 112464. [Google Scholar] [CrossRef]
- Catalano, J.; Guazzone, F.; Mardilovich, I.P.; Kazantzis, N.K.; Ma, Y.H. Hydrogen Production in a Large Scale Water–Gas Shift Pd-Based Catalytic Membrane Reactor. Ind. Eng. Chem. Res. 2012, 52, 1042–1055. [Google Scholar] [CrossRef]
- Peters, J.F. Pyrolysis for Biofuels or Biochar?—A Thermodynamic, Environmental and Economic Assessment. Ph.D. Thesis, Universidad Rey Juan Carlos, Móstoles, Spain, 2017. [Google Scholar] [CrossRef]
- Patel, M.; Kumar, A. Production of renewable diesel through the hydroprocessing of lignocellulosic biomass-derived bio-oil: A review. Renew. Sustain. Energy Rev. 2016, 58, 1293–1307. [Google Scholar] [CrossRef]
- He, Z.; Wang, X. Hydrodeoxygenation of model compounds and catalytic systems for pyrolysis bio-oils upgrading. Catal. Sustain. Energy 2012, 1, 28–52. [Google Scholar] [CrossRef]
- Mani, S.; Tabil, L.G.; Sokhansanj, S. Grinding performance and physical properties of wheat and barley straws, corn stover and switchgrass. Biomass-Bioenergy 2004, 27, 339–352. [Google Scholar] [CrossRef]







| Parameter | Unit | References |
|---|---|---|
| C | 50.7 w% db | [26] |
| H | 5.9 w% db | [26] |
| N | 0.2 w% db | [26] |
| O | 43.0 w% db | [26] |
| S | 0.005 w% db | [26] |
| Cl | 0.005 w% db | [26] |
| Volatiles | 85.4 w% db | [26] |
| Fixed C | 14.6 w% db | [26] |
| Ash content | 0.2 w% db | [26] |
| Water content | 7.2 w% db | [26] |
| LHV (dry) | 18.9 [MJ kgdb−1] | [26] |
| LHV (moist) | 17.4 [MJ kg−1] | [26] |
| Cellulose | 44% | [27] |
| Hemicellulos | 24% | [27] |
| Lignin | 32% | [27] |
| Reaction | Reaction Name | Heat of Reaction | Reaction Number |
|---|---|---|---|
| Heterogeneous reaction | |||
| C + 0.5 O2 → CO | Char partial combustion | (−111 MJ kmol−1) | (R1) |
| C + H2O ↔ CO + H2 | Water–gas | (+172 MJ kmol−1) | (R2) |
| 2 CO ↔ CO2 + C | Boudouard | (+172 MJ kmol−1) | (R3) |
| Homogeneous reactions | |||
| H2 + 0.5 O2 → H2O | H2 partial combustion | (−283 MJ kmol−1) | (R4) |
| CO + H2O ↔ CO2 + H2 | Water–gas shift | (−41 MJ kmol−1) | (R5) |
| CH4 + H2O → CO + 3H2 | Steam-methane reforming | (+206 MJ kmol−1) | (R6) |
| CaO + CO2 ↔ CaCO3 | Carbonation | (−179 MJ kmol−1) | (R7) |
| SYNGAS,0 (Out of SEG) | SYNGAS,1 (Out BEDREACT) | SYNGAS,2 (Out CANDLE) | SYNGAS,3 (Out H2S Removal) | SYNGAS,4 (Out HCl Removal) | |
|---|---|---|---|---|---|
| H2 [%vol,dry] | 80.17 | 80.17 | 83.87 | 83.87 | 83.87 |
| CO [%vol,dry] | 6.24 | 6.24 | 11.5 | 11.5 | 11.5 |
| CO2 [%vol,dry] | 1.18 | 1.18 | 0.98 | 0.98 | 0.98 |
| CH4 [%vol,dry] | 12.45 | 12.45 | 3.6 | 3.6 | 3.6 |
| C6H6 [g/Nm3] | 21.15 | 5.28 | 1.56 | 1.56 | 1.56 |
| C7H8 [g/Nm3] | 8.60 | 1.63 | 0.45 | 0.45 | 0.45 |
| C10H8 [g/Nm3] | 11.57 | 2.05 | 0.62 | 0.62 | 0.62 |
| H2S [ppm] | 1200 | 200 | 180 | 0.04 | 0.04 |
| HCl [ppm] | 750 | 105 | 100 | 0.03 | 0.03 |
| Reaction | Reaction Number |
|---|---|
| Chrysene + 3 H2 → Naphthalene + M-Xylene | (R8) |
| Chrysene + 10.1 H2 → 0.35 Benzene + 0.25 Dodecane + 0.32 Isopropylbenzene + + 0.32 Methylcyclohexane + 0.32 Ethane + 0.33 Toluene + 0.43 Undecane + 0.1 CH4 | (R9) |
| Chrysene + 11 H2 → Cyclohexane + Bicyclohexyl | (R10) |
| Chrysene + 13 H2 → 0.35 N-octadecane + 0.33 Isopropylcyclohexane + + 0.33 N-nonane + 0.32 Cyclopentane + 0.32 N-tridecane | (R11) |
| Chrysene + 14.25 H2 → 0.5 Pentane + 0.5 N-octane + 0.25 Methylnonane + + 0.5 N-pentadecane + 0.5 Propane | (R12) |
| Chrysene + 15.6 H2 → Butane + 0.8 Tetradecane + 0.2 N-hexane + 1.6 CH4 | (R13) |
| Pre HDO | Post HDO | |
|---|---|---|
| C (% wt.) | 56.2 | 85.5 |
| H (% wt.) | 6.1 | 14.3 |
| O (% wt.) | 37.7 | 0.2 |
| LHV (MJ/kg) | 18.5 | 46.3 |
| Pretreatment | Pyrolysis | Gasification | HDO | Total | |
|---|---|---|---|---|---|
| No pyro | 0.53 | 0 | 7.19 | 0 | 7.72 |
| 300 °C | 0.53 | 0.65 | 4.74 | 1.49 | 7.41 |
| 350 °C | 0.53 | 0.93 | 3.19 | 2.38 | 7.02 |
| 400 °C | 0.53 | 1.03 | 2.68 | 2.32 | 6.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartolucci, L.; Bocci, E.; Cordiner, S.; De Maina, E.; Lombardi, F.; Marcantonio, V.; Mele, P.; Mulone, V.; Sorino, D. Biomass Polygeneration System for the Thermal Conversion of Softwood Waste into Hydrogen and Drop-In Biofuels. Energies 2023, 16, 1286. https://doi.org/10.3390/en16031286
Bartolucci L, Bocci E, Cordiner S, De Maina E, Lombardi F, Marcantonio V, Mele P, Mulone V, Sorino D. Biomass Polygeneration System for the Thermal Conversion of Softwood Waste into Hydrogen and Drop-In Biofuels. Energies. 2023; 16(3):1286. https://doi.org/10.3390/en16031286
Chicago/Turabian StyleBartolucci, Lorenzo, Enrico Bocci, Stefano Cordiner, Emanuele De Maina, Francesco Lombardi, Vera Marcantonio, Pietro Mele, Vincenzo Mulone, and Davide Sorino. 2023. "Biomass Polygeneration System for the Thermal Conversion of Softwood Waste into Hydrogen and Drop-In Biofuels" Energies 16, no. 3: 1286. https://doi.org/10.3390/en16031286
APA StyleBartolucci, L., Bocci, E., Cordiner, S., De Maina, E., Lombardi, F., Marcantonio, V., Mele, P., Mulone, V., & Sorino, D. (2023). Biomass Polygeneration System for the Thermal Conversion of Softwood Waste into Hydrogen and Drop-In Biofuels. Energies, 16(3), 1286. https://doi.org/10.3390/en16031286